January & February 2024: Complexity in Ecology
By Dr Alexey Larionov, Co-Investigator from Cranfield University
Attention to ecological complexity has been growing recently, because of concerns about anthropogenic impact on environment. The hope is that “sufficiently complex” ecosystems will be able to adapt and maintain the key functions needed for human wellbeing. The fear is that the current technologies are so advanced that they can cause (and may be already causing) a mass extinction of species on Earth, reducing the biodiversity beyond a tipping point leading to non-reversable consequences.
In this context, “ecological complexity” is implicitly defined from the functional perspective: as the ability to maintain a good habitat for humankind. Intuitively it is linked to some level of biodiversity (i.e. multiple interacting species), some dynamic behaviour (ability to maintain a dynamic stable state, or make sudden non-linear changes), and some spatial organisation. While there is some conceptual consensus about the importance of all these aspects, there is no clarity about how to measure them, and even less clarity about how to integrate the multiple measures into a unified ecological complexity index.
Diversity
The measures of biodiversity are notoriously diverse: Shannon entropy index, Pielow evenness index, Chao index, Simpson’s index, Faith PD index etc (to name just a few of the popular Alpha diversity metrics). By design, different metrics capture different aspects of diversity: richness, evenness, and phylogenetic relations between the species within the ecosystem. Figure 1 shows an example of different alpha-diversity indices.
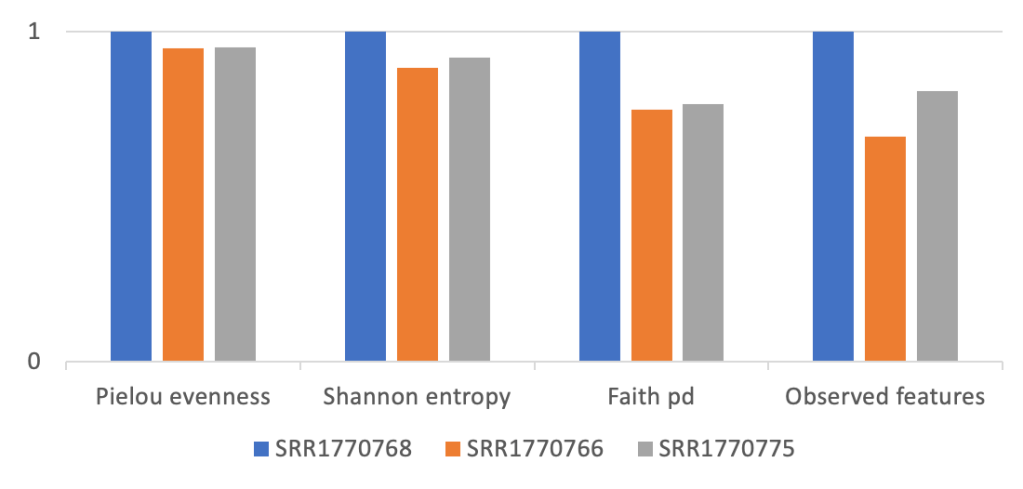
Figure 1: Example of different alpha-diversity indices (to enable comparison y-axis is shown in relative units, calculated for three soil samples selected from data published by Leff et al 2015, www.pnas.org/cgi/doi/10.1073/pnas.1508382112)
Connectivity
The mathematical theory of networks (based on graph theory) is widely used for analysis of connectivity between species in ecosystems. Quantitative parameters capturing network architecture include size, density, degree, path(s) length between elements, clustering etc. Importantly, biological interpretation of these mathematical parameters depends on the type of connections reflected by the network. Thus, interpreting trophic networks is reasonably straight-forward, e.g. for highlighting key species or vulnerabilities in the food chain. At the same time, physical interactions, or correlations in abundancy (commonly used in microbiome analysis, Figure 2) have less clear biological interpretations.
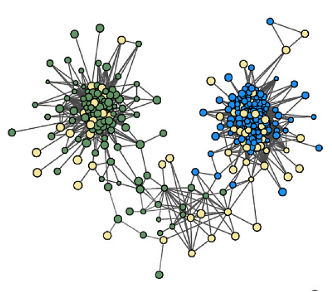
Figure 2: Example of a microbial co-occurrence (abundancies correlation) network in soil green: upper layer, blue: lower layer, yellow: in both layers (fragment of fig 4 from Guseva et al 2022, https://doi.org/10.1016/j.soilbio.2021.108534 )
Dynamics
Ordinary Differential Equations (ODEs) are widely used to describe dynamic interactions within the prey-predator or grass-grazer systems. For instance, Lotka-Volterra equations have been used for dynamic modelling over a century. ODEs predict species abundancies over time based on their rates of change. Typically, for a stable ecosystem it predicts some oscillation of species abundancies over time (Figure 3). Also, ODEs could be used to predict tipping points, when the system state may dramatically change (possibly switching to another stable state …).
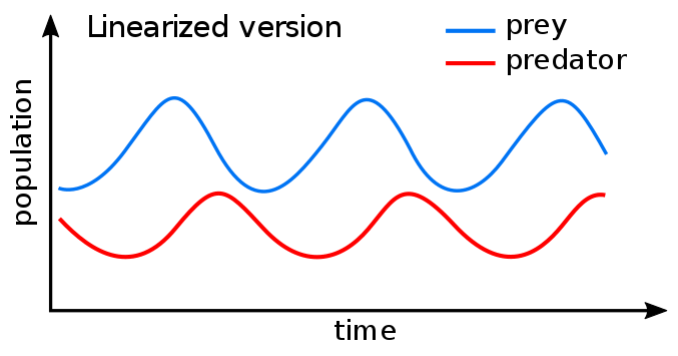
Figure 3: Example of a Lotka-Volterra solution for a prey-predator system (https://en.wikipedia.org/wiki/Lotka-Volterra_equations)
Spatial organisation
Spatial organisation (sometime called structural diversity) includes both biotic and abiotic components of the ecosystem. For instance, a forest structural diversity could be captured by spatial distribution of the heights and diameters of trees, which could be a better predictor of biomass than the species diversity (LaRue et al 2023, https://doi.org/10.1002/fee.2586 ). Other examples of spatial organisation are the shape of a coastal line, or the shape of coral reefs. Again, there are different approaches to measure the complexity of such shapes. Fractal dimensionsare very popular to describe spatial organisation and complexity, having a deep mathematical foundation. At the same time, fractals could be a good illustration of a risk posed by using (and misusing) complex mathematical concepts in ecology: when mathematicians and ecologists do not fully understand each other. Thus, it is often assumed by non-mathematicians that fractals require some sort of scaling self-similarity. However, fractal dimension can be defined for any shape, without requirement for self-similarity. For instance, it could be calculated for a coastline (Fig 4).
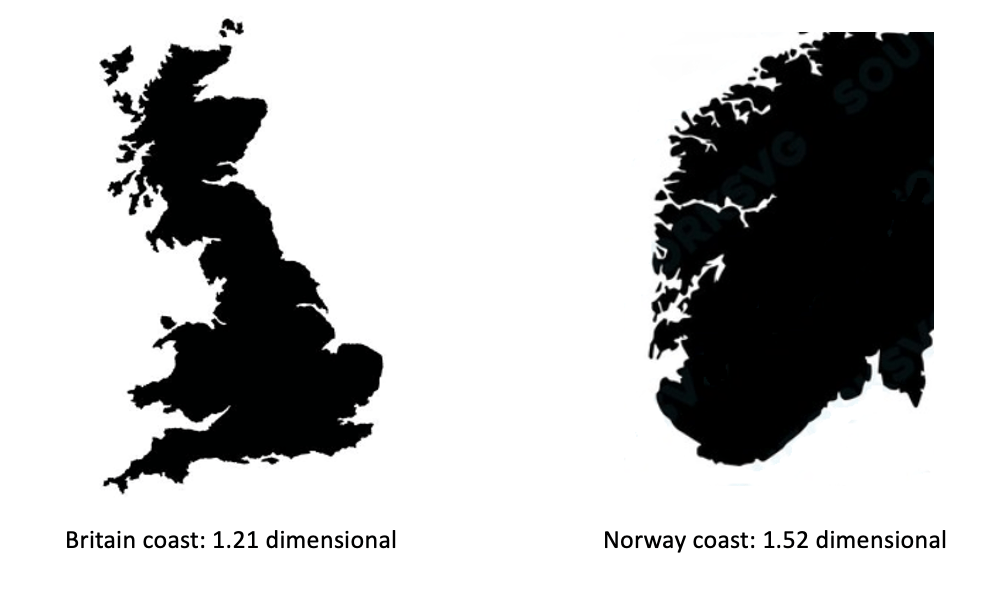
Figure 4: Fractal dimensions of coastlines
You may watch a nice 20 min lay introduction about fractal dimensions here: https://www.youtube.com/watch?v=gB9n2gHsHN4 . Unfortunately, the meaning of fractal dimensionality is disconnected from the conventional topological dimensionality, which makes it quite mysterious and even confusing for a non-mathematician: how something could be 1.5 dimensional? More intuitive measures could be used to demystify the roughness of lines and surfaces: such as rugosity (e.g. the length of contour over its longest linear projection) or surface per volume (e.g. for the coral reefs). In many cases, such measures may numerically correlate to fractal dimensionality, at the same time having the benefit of clear physical interpretation.
Statistical modelling
Standard statistical modelling (descriptive statistics, regression, classification, clustering etc) is also widely used to describe and analyse ecosystems. More advanced statistical techniques include Structural Equation Modelling(SEM), which attempts to analyse “latent variables” that cannot be measured directly. Importantly, the SEM framework allows researchers to describe the hypothetical structure of an ecosystem as a graph, connecting latent variables with some measurable parameters (see example on Figure 5).
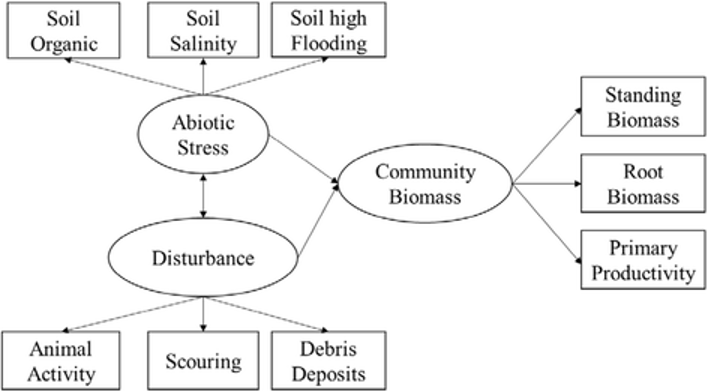
Figure 5: Example of SEM graph: encircled components are latent (non-measurable) variables; rectangular components are measurable parameters; arrows direction shows assumed causality (Fan et al 2016, https://doi.org/10.1186/s13717-016-0063-3, Fig 2)
Along with the graph, SEM accepts the values of the measured parameters. Then, SEM generates and analyses a system of regression equations to establish whether the hypothetical structure is consistent with the data. A simple introduction to Structural Equation Modelling could be found here: https://www.youtube.com/playlist?list=PLzv58M2GAfm4CF5-7GGDYStx9SzyhFmgK
Practical applications
So far, we discussed selected examples of the methods used for quantitative analysis of ecological complexity in science. However, along with scientific exploration, measuring ecological complexity is also needed for regulatory purposes. Governmental policies like Biodiversity Net Gain (https://www.gov.uk/government/collections/biodiversity-net-gain ) or Sustainable Farming Initiative (https://farming.campaign.gov.uk) have to define specific practical procedures to monitor complexity of ecosystems. This requires simple common-sense methods: clear for policymakers, for society, and for implementation. The scientific methods, such as fractals, differential or structural equation models are not very good for such a context. This is why the government policies are focusing on diversity (or even on selected indicator species) rather than on complexity.
A simple practical approach to ecological complexity measurement was proposed by the RestREco team in a recent paper, which considers complexity at the landscape-level (Bullock et al 2022, https://doi.org/10.1111/ecog.05780 ). Conceptually, the paper links complexity to resilience: ability to sustain or reach a stable diverse state after disturbance(s). Practically, the paper describes complexity at landscape level as a set of interconnected diverse sites (Figure 6). The suggested empirical measure of ecological complexity is defined as “the number of components in a system and the number of connections among them”.
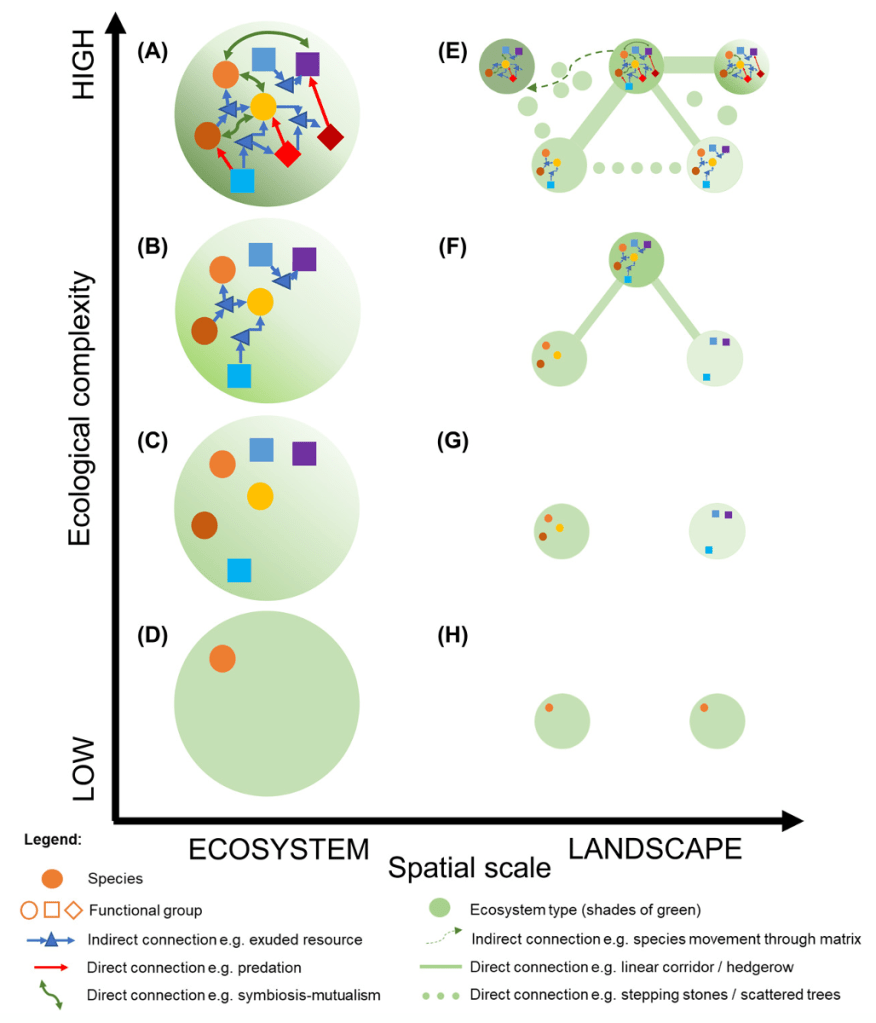
Figure 6: Ecological complexity at landscaper level (Bullock et al 2022, https://doi.org/10.1111/ecog.05780, Fig 2)
Although details of how to count the connections and how to define and count components is still open for discussion, such an approach could be much closer to practical application than the complex scientific methods described before.
Approaches currently considered by the RestREco team
The RestREco team considers multiple approaches to tackle ecological complexity during restoration. First of all, we capture a wide range of parameters, such as multiple soil metrics (including bacterial and fungal communities and many physical/chemical parameters), plant and invertebrate species, structural diversity (in forest sites), soundscape, remote imaging etc. We then use standard statistical methods to evaluate the effects of the studied factors (initial state, proximity of non-affected sites, management etc) onto the multiple measured parameters. Analysing the age of restoration may give us indications about the dynamics of different parameters during restoration. The field-specific methods will be used for each type of data, e.g. multiple alpha and beta diversity metrics and functional estimates of microbial community for soil (Figure 7). For integrating multiple types of data, we currently evaluate the SEM framework. At the same time, from the applied perspective, we may explore approaches outlined above for the landscape-level complexity assessment (depending on the availability of data).

Figure 7: Sam analysing microbial diversity
December 2023: RestREco at BES Annual Meeting in Belfast!
Some of the team were at the Annual meeting of the British Ecological Society in Belfast this week. There were lots of interesting talks, posters, workshops, and of course, lots of conversation over coffee and drinks!
Isabella Tree gave a very inspirational talk about Knepp, one of our project partners and the site of both ‘woodland’ and grassland wildcards (search ‘wildcards’ below for more details on these!). RestREco’s Prof James Bullock (UKCEH) has started up a new BES special interest group (SIG) on Rewilding, along with Prof Nathalie Pettorelli from ZSL. The aim of this SIG is to boost information availability and knowledge transfer on rewilding, supporting the emergence of new collaborations and innovation in rewilding science and practice, in the UK and globally (see here for more details).
And finally, Ben McCarthy from National Trust presented a poster on RestREco at the poster session. Have a read of it below.
Merry Christmas from RestREco and see you in the New Year for more updates!
November 2023: An update from the grassland team
By Maico Geert Weites, Research Technician at UKCEH
After a long summer and autumn of fieldwork in glorious and less glorious weather the grassland team has finished their fieldwork for the year. The flowerheads have been collected, the soil cores taken, soil moisture measured, and rain shelters dismantled.

Horses observing us dismantling the rain shelters. Melanie in the background.
This does not mean however, that we can rest our laurels! Currently we are busy with finishing up the identification process of all the invertebrate samples we have collected in this as well as in past field seasons.
During the field season we put out pitfall traps on our field sites in order to catch predatory beetles to analyse their diet by using eDNA. This should tell us what these predators feed on and contribute to understanding the composition of the food webs. The dirty work is now done and all the beetles have been identified, sorted, and are ready to be have their gut content examined soon! Pitfall traps turn up many beetle species that we struggle with catching with other methods. Many ground beetles are nocturnal and are fast runners. So rather than running around grasslands at night we just burry some cups in the soil and wait for the beetles to run in. It’s astounding how many beetles can turn up in these traps even after just a few nights. Pterostichus madidus and Calathus fuscipes were our most commonly caught beetles but we also came across some more exciting species such as Licinus depressus, the colourful Panagaeus bipustulatus, and the large bronze Carabus monilis.
Our most common beetle that was not a ground beetle was the Devil’s Coach-horse (Ocypus olens). These large predators are the largest rove beetles in the British Isles and although their elytra are very short, their large wings are neatly folded up underneath them and they are very good flyers.


Left: Specimens being pointed for future reference. Right: One of the many Devil’s Coach-horse beetles (Ocypus olens) from the pitfall traps. This specimen was mounted with its wings displayed.
The biggest identification job however are the suction samples. These samples often contain well over a hundred species and also contain a whole load of sand, seeds, leaves, pollen, and dung. After filtering out the invertebrates we get to the identification game. So far we have filled our spreadsheet with over 7000 rows of species from different samples, comprising many hundreds of species. Of our 134 suction samples we only have 8 left to do, so we’re almost there! Some of our recent interesting finds from these samples include the rare leafhopper Hephathus nanus that specialised on Dward Thistle (Cirsium acaule), the introduced small American money spider Mermessus trilobatus, and the leafhopper Aphrodes diminuta, best identified by the male genitalia.




Clockwise from top left: The leafhopper Aphrodes diminuta, best identified by the male genitalia; Male genitalia of Aphrodes diminuta; The male palp of spider Mermessus trilobatus; The rare leafhopper Hephathus nanus.
Photo credits: Maico Geert Weites
October 2023: Decoding Ecosystem Resilience From Space
By Will Rust, Research Fellow at Cranfield University
Hello! I’m Will Rust, and while many of my colleagues in the RestREco project are out exploring, conducting lab experiments, and getting their hands dirty to understand ecosystem resilience, I have a slightly different perspective. My job is to track ecosystem resilience from space!
Unlike the tangible experiments on the ground, I rely on satellite measurements of vegetative growth. By examining the amount of light that is reflected by leaves and other plant biomasses at different stages of growth, or at different levels of health, we can measure the vigour of vegetative growth: the Normalized Difference Vegetation Index (NDVI). A key benefit of this approach is that we can measure vegetation health over the course of years or decades.
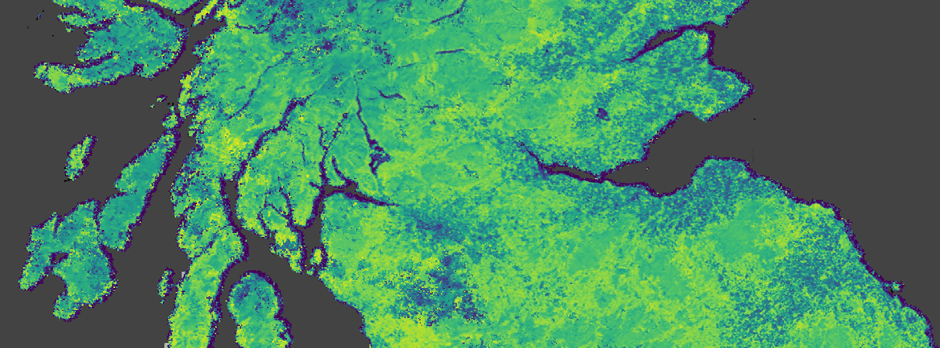
Image taken from Google Earth Engine. Data shows NDVI from the MODIS 16-day composite dataset.
With decades of NDVI data available to us, we can start to identify patterns and changes in an ecosystem. The central goal of the RestREco project is to decode ecosystem resilience. At its core, resilience refers to an ecosystem’s ability to withstand shocks and return to its original state efficiently. Just as a sick animal might recover more slowly from an illness, unhealthy ecosystems also suffer from slower recovery times. This is called “Critical Slowing Down”. And if an ecosystem slows down too much, any external influence might push it into a different state: Ecosystem Collapse. This can be a biodiverse woodland collapsing to a scrubland with poorer biodiversity. It is also incredibly difficult for these ecosystems to then return to a high-biodiversity state.
Thankfully, signals of Critical Slowing Down can be found hidden within long-term NDVI data. By observing the autocorrelation in NDVI, we can gauge this ‘system slowness’. And when we see autocorrelation suddenly increasing, we can relate this to the snowballing effect of reducing ecosystem health and predict an imminent collapse… before it happens!
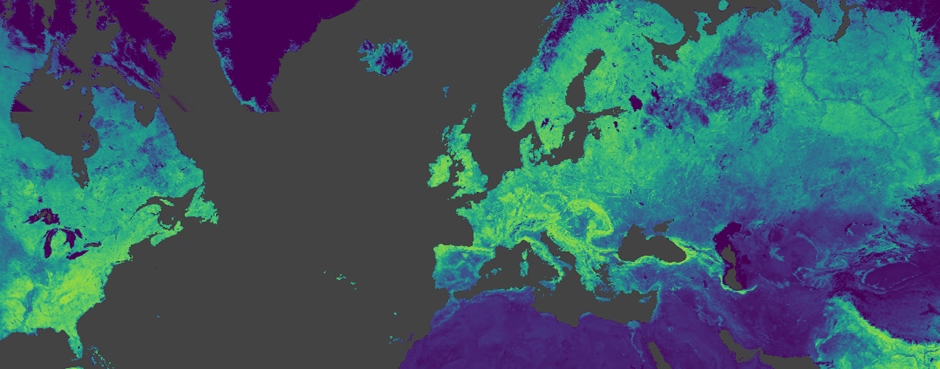
Image taken from Google Earth Engine. Data shows NDVI from the MODIS 16-day composite dataset.
However, things aren’t this simple. Previous studies have designed models to detect these tipping points in ecosystems with known collapse records. But, they’ve mostly focused on regions like Californian woodlands, where the skies are cloud-free for most of the year. The UK is a little different, and cloud causes a major problem with measuring anything from space.
So, the crux of my work now focuses on devising algorithms that can effectively filter out clouds from satellite imagery. It’s essential to ensure we can confidently measure NDVI in the UK and use it to predict potential ecosystem collapses.
The dream is to wield these models and pinpoint any vegetated location on Earth, measuring its resilience. It’s an ambitious dream, not without challenges, but we are making good progress towards cloud-free space data!
Here’s to understanding our ecosystems better, from hundreds of miles above!
September 2023: Soil Analysis at Cranfield
By Sam Hibdige, Research Fellow
Sam here with a soil update! As the freshly minted bioinformatician it’s my job to look at the microorganism communities in the restoration sites across the project. I will mainly be looking at bacteria and fungi present in the soil in both percentage composition and their functionality. Unlike some organisms, functionality can vary significantly within a species of microorganism, some communities for example might have completely different tolerances to a range of environmental factors, so it’s important to understand functionality in relation to species composition. The statistic that blew me away is that the bacteria E.coli has a core genome of only 2,200 genes whilst the pangenome (the bit which can vary between communities) has up to 13,000 genes! With that amount of variation you can see how different communities of E.coli could be up to completely different things.
How is this done? The oldest, most tried method of sampling is via amplicon sequencing. Sequencing the whole genome of an organism is expensive and time consuming, so if you are only interested in what is present in a community, you just sequence a small part of the genome known as an amplicon. When choosing an amplicon, you are looking for a gene or sequence present in all species (some genes are only present in groups of related species), it must be relatively conserved to allow it to be comparable but not too conserved you can’t tell different species apart. Luckily for me, several amplicons for bacterial and fungi diversity have been established over the years so I don’t need to re-invent the wheel. As bacteria and fungi are two distantly related groups, you use a different amplicon for each.


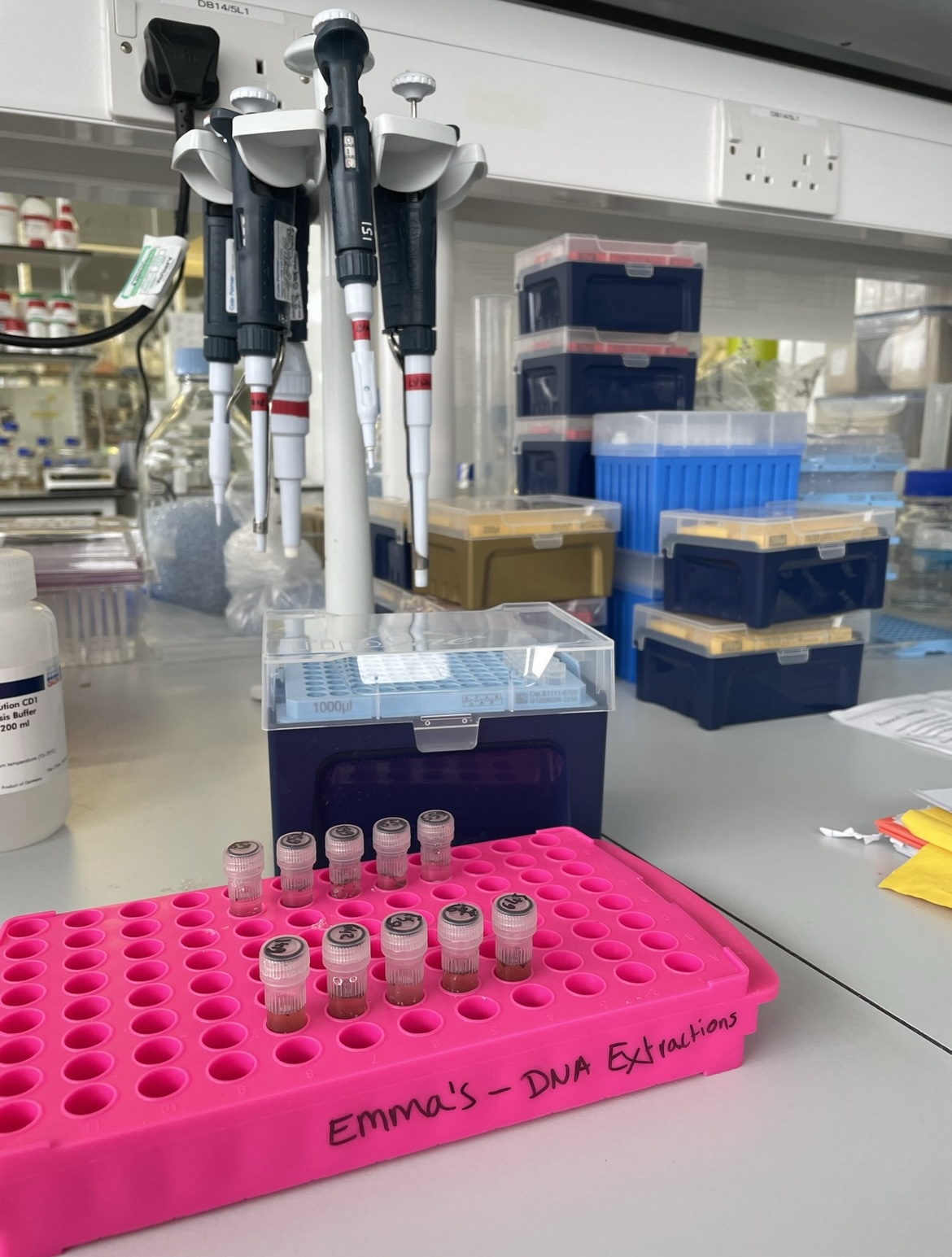


Soil samples in the lab must go through various stages of processing and filtration to extract the DNA we’re looking for
There are a lot of steps before I get hold of this sequencing data including extracting the DNA in the lab. This has proved particularly difficult with some of the soil types that have been sampled, as you can imagine DNA extraction required specific conditions at each step and the presence of deposits such as chalk can really mess with the buffering process! When I get the data it’s a series of “reads”. Reads are sequences (in this case the amplicons) that have been amplified to a level to where they can be detected. As they are amplified at the same time, they are proportional to one and other. This means once you identify which reads belong to which species, you have a proportional abundance of each species in the community you sampled!
It’s a bit more complex than that of course, you must go through several cleaning steps to through away bad “reads”. Even though the sequencing methods get better and better every year, you are always going to have erroneous results. Ironically as the methods for detecting erroneous reads get better and better, the need for it decreases as the sequencing technology gets better and better. You also must be able to identify what is in your sample. In days gone by, only microorganisms you could grow in the lab could be identified, a lot of them don’t like to do this however which of course makes my life difficult. You may have heard we have no idea how many species of insects exist and are constantly discovering new species every year. The same is true with microorganisms, the problem is no one agrees on how to put them into taxonomy but that is a blog post for another time. The whole point I am alluding to is a lot of the time we don’t know what we are looking at. The good news is that if we can’t identify it at the species level you can at least give it a genus, family, order, class etc. Once all of that is done, we can finally begin to look for some differences between our different woodlands and grasslands and apply some statistics!
What I want to know is whether we are seeing different microorganisms present in soils from different land use practices, whether some are present across all restoration sites and whether it is functionality that is unifying as opposed to species. Watch this space, I’m told the sequencing data is not far off…
August 2023: Fun at Foulshiels!
This month, project partners the Woodland Trust tell us about the fun events they’ve hosted in one of our Scottish research sites: Foulshiels Wood. For more on the Woodland Trust, and to find events in your area, please visit www.woodlandtrust.org.uk
By Hannah Patterson, Woodland Trust Site Manager for Central Scotland
Foulshiels is unlike any of the other 16 sites that the Woodland Trust manages within West Lothian. It stands apart in both its history and diversity of habitats.
When the coal mining ceased on this site in 1958, the large spoil heap remained and has since been transformed as it has been reclaimed by nature. Despite covering just under 30 hectares, Foulshiels is now an intimate mixture of grassland, heathland, wet woodland, areas of dense conifers, native broadleaves and thickets of scrub. The bing of Foulshiels stands out as an important visual feature in the landscape as it is surrounded by grassland and fields on all-sides and the town of Stoneyburn to the south.
Beyond the bing, another subtle reminder of the site’s industrial past, is the railway line that skirts across the north of the site. Where once goods were carried to and from the mines, this line has now morphed into the path network, enabling visitors to journey through the site and beyond. In early 2022, extensive path upgrade works were completed from each entrance across a large loop, leading into the Core Path from Stoneyburn to East Whitburn. By following the flat, level route of the old railway line, visitors travel through the varied scenery the site has to offer with ease. The paths also climb to the top of the bing providing beautiful vistas of the surrounding landscape. Providing a combination of flat sections and inclines, the upgraded paths have been greatly appreciated by the wider community, including those that participate in the annual fun run at Foulshiels.
The site is within in walking distance to two primary schools- Stoneyburn Primary School and Our Lady’s RC Primary School. The Woodland Trust commissioned Rowanbank, an environmentally focused educational theatrical group, to conduct workshops in these schools. Rowanbank then lead magical woodland walks at Foulshiels in June 2023. These were wonderfully received- not only raising awareness of improvements to the paths making the site more accessible for visitors but also providing more confidence to local teaching staff to facilitate outdoor learning for their classes.

Rowanbank characters leading the Magical Woodland Walk at Foulshiels 2023- Photo credit Hannah Patterson
At the beginning of July, we held our first BioBlitz at Foulshiels in collaboration with the RestREco Woodland Team at the University of Stirling. Unfortunately, the Scottish weather wanted to make its presence known immediately which put a bit of dampener on the day. Nevertheless, a few very enthusiastic locals braved the rain to look for all the wildlife that call Foulshiels home, such as frogs, reg-legged shield bugs and song thrushes. Interesting floral species spotted include the greater butterfly orchid (Platanthera chloranta), broadleaved helleborine (Epipactis helleborine) and common twayblade (Neottia ovata). Some visitors shared stories of the history of the site passed down through relatives that worked in the mines or from their own discoveries!

Bioblitz at Foulshiels 2023- Photo Credit: Hannah Patterson
Foulshiels is a perfect example of why urban woodlands are so important – acting as an essential refuge for wildlife and humans alike. They provide the feeling of being far away from the hustle and bustle of modern life whilst being a stones-throw from the bus stop and the corner shop. The Woodland Trust is proud to look after Foulshiels and plan to work with the local community to capture its interesting historical and environmental findings for information boards about the area. This engagement will hopefully inspire more people to appreciate and want to protect these urban woodlands for future generations to enjoy.
July 2023: Glorious Grasslands: A summer of fieldwork
By Melanie Shears, Research Technician at UKCEH
Following an onerous May building rain shelters in some very wet weather, the grassland team have been enjoying working in some sunnier weather over the past few months. Throughout June and July our fieldwork has focused on quantifying key ecosystem functions in 19 grassland sites, including pollination, primary production, and predation. These functions are being investigated using various field experiments.
Pollination rates are being measured using bird’s-foot trefoil (Lotus corniculatus) and rough hawkbit (Leontodon hispidus). With the use of organza bags, pollinators are being excluded from unopened flower buds, whilst leaving unbagged flowers accessible for pollination. Both bagged and un-bagged flowers will soon be harvested (once they have matured), and the seeds will be counted for comparison. We have also been conducting pollinator surveys along 50m transects on each of the field sites. This involves counting the invertebrates and noting which flowers they are visiting, as well as noting all the floral resources along the transect.


Left: Maico completes a botanical survey. Right: Bagged flower head of Leontodon hispidus
Throughout May and June, exclusion cages were set up on each of the sites to measure primary production. A small area of vegetation was cut and then covered with an exclusion cage which prevented livestock and deer from grazing the plant matter encased inside. The vegetation was left to grow undisturbed over a period of several weeks before being cut and collected. Samples will now need to be dried and weighed to measure the plant biomass and growth.
We are currently collecting predatory invertebrates (mainly wolf spiders and ground beetles) to send off for DNA metabarcoding of their gut content. The purpose of this is to assess what diversity is in the predators’ diets and contribute to building food webs. Using pitfall traps to collect the predatory invertebrates has yielded the highest number of samples, although we also experimented with using sweep nets and suction sampling. It will be exciting to see what the DNA metabarcoding reveals when the samples have been sent off!
June 2023: Trees for Climate, Biodiversity and People Conference
By Dr Emily Waddell, Postdoctoral Researcher & Ross Barnett, Research Technician
Last week some of the woodland folk headed down to Kent for the joint British Ecological Society, University of Kent and Woodland Trust conference ‘Trees for Climate Change, Biodiversity and People’. The two-day event was a mixture of posters and talks, which were split into 3 sessions covering the following themes: Culture, Heritage & History of Trees, Tree Health and 30×30 and Landscape Restoration. Within each themed session we heard three invited talks followed by a series of shorter talks, finishing with a Q&A panel of the invited speakers. RestREco’s woodland research technician Ross presented ‘The Sound of Restoration’ at the 30×30 and Landscape Restoration session.
Day 1 kicked off with the plenary by Dr Forrest Fleischman from the University of Minnesota, who discussed among many things the successes and failures of forest restoration from his research in Northern India. Forrest emphasised in his talk that trees are the result of social and political relationships, and the involvement of local community is crucial to the success of forest restoration. The importance of trees and forests to people and communities continued into the first theme ‘Culture, Heritage & History of Trees’. Dr Coralie Mills of Dendrochronicle discussed how trees are deeply rooted in our histories and the importance of understanding the history of trees and forests in order to protect them. Prof Aliyu Salisu Barau of Bayero University continued this by discussing the importance of local trees to the community of his hometown Kano, Nigeria. Aliyu told us heart-warming stories of sharing fruits and medicines with neighbours and playing in nature, before discussing the loss of trees from these neighbourhoods in recent years. Finally, Prof Zoe Davies from the University of Kent gave us a really interesting talk on the impact of woodland biodiversity on human wellbeing. It wasn’t a surprise that trees provide multiple benefits to people! In the shorter talks we heard about tiny forests being planted in the UK (based on Japanese Miyawaki forests), the history of elm decline in the UK, the culture of polarisation between scientists, foresters and conservationists (by RestREco’s Woodland Trust partner Dr Chris Nicols), the aesthetics of urban treescapes and people’s wellbeing and finally the importance of language when conserving forests.


Presentations by Prof. Aliyu Barau (Bayero University) and Dr. Forrest Fleischman (University of Minnesota)
The next session on ‘Tree Health’ started with an enthusiastic talk from Woodland Trust’s Rebecca Gosling, who gave an overview of the exponential increase in number tree pests and pathogens recorded in the UK, monitoring efforts and control strategies. This was followed by the role of social science and humanities in understanding both tree health and biosecurity from Dr Julie Urquhart of University of Gloucestershire. Finally, Prof Lucio Montecchio from the University of Padova discussed the role of good communication in monitoring tree health and biosecurity, and stressed that known pests and pathogens must be studied before their arrival to get ahead of future outbreaks. These were followed by a range of talks discussing specific tree pests and pathogens, in particular ash dieback and oak processionary moth, the resilience of trees to these pests and pathogens, and the role citizen science can play in monitoring outbreaks. The first day ended with a poster session and a barbecue – a lovely chance to meet fellow scientists, conservationists and practitioners with good food and live music!
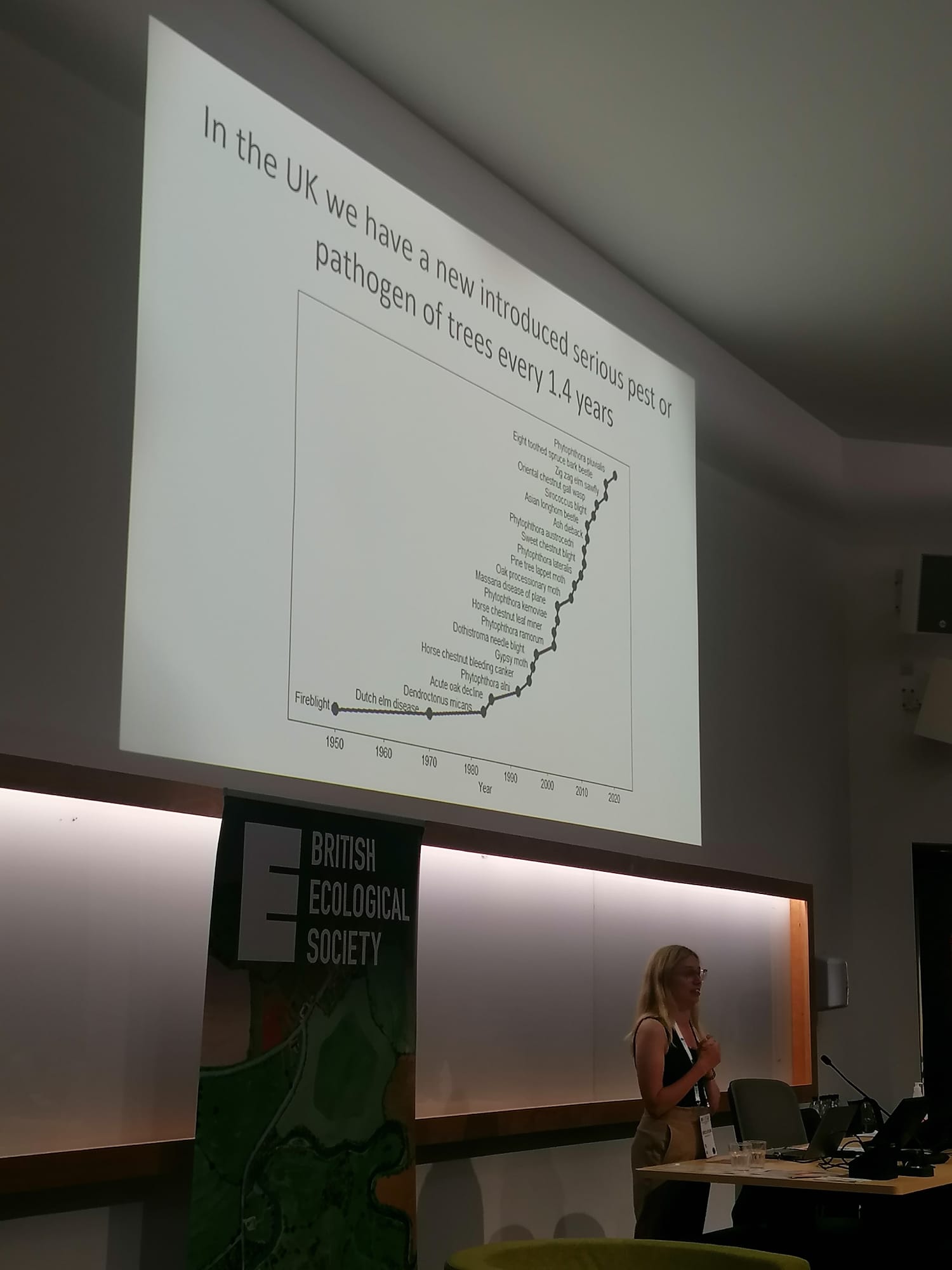

Presentations by Rebecca Gosling (Woodland Trust) and Prof. Zoe Davies (DICE, University of Kent)
On the second day of Trees4CBP, we attended the session on 30×30 and Landscape Restoration. The session was hugely informative, with three plenary talks on a range of topics relating to conservation and restoration. Dr Andy Stott, UK Lead Negotiator to COP15, opened the session telling us about the Global Biodiversity Framework and it’s targets. Next, Dr Cat Scott, Director of the Leeds Ecosystem, Atmosphere and Forest (LEAF) centre (University of Leeds) gave a talk on work LEAF are carrying out across several projects, with an emphasis on the importance of monitoring restoration projects to ensure maximum benefit for climate and biodiversity. Nick Hanley, Professor of Environmental Economics at the University of Glasgow led the last plenary. Nick presented some findings from a collaboration between Glasgow and Stirling, using the WrEN dataset to model biodiversity benefits of economic incentive programs for restoration.
The rest of the session had talks from scientists from academia, the RSPB, Woodland Trust and more. We heard talks discussing where best to plant new woodland, the role of treescapes in achieving net-zero and the need to balance restoration and social progress goals. I was very excited to have had my own talk accepted: “The sound of restoration: What can acoustic indices tell us about complexity in restored woodland?”. This was my first (in-person) conference presentation and it was great to present some preliminary findings from our acoustic monitoring in woodland sites. We are investigating how acoustic indices respond to ecosystem complexity and landscape variables. Acoustic indices, developed in the last two decades or so, provide scores that respond to attributes of a soundscape. These preliminary investigations I presented show that some indices do respond to variation in woodland structural complexity, however index values show a lot of variation across the day, and there is much more still to untangle!


Presentations by Dr Chris Nichols (Woodland Trust, RestREco Project Partner) and Ross Barnett (Woodland Research Technician)
The day closed with a wonderful dose of positivity – a presentation by the charity Action for Conservation. Action for Conservation work provide a platform for young people in the UK to get hands-on experience planning and working on active nature recovery and conservation projects. One of the young people they work with, Dom McWilliams, led most of the presentation, and it was great to hear how conservation work had helped him become more confident and feel more positive about the climate crisis.
May 2023: From the Field: Drought experiments in chalk grasslands
By Dr Ben Woodcock, Co-Investigator & Maico Weites, Research Technician
A big part of the RestREco project is trying to understand how complexity in ecological systems – in the case of this project established though restoration – can improve the resilience of these systems to future environmental change. Are biological systems with diverse species and complex webs of feeding relationships better able to resist perturbations, such as the droughts we saw in 2022, when compared to far simpler ones? To answer this question we have been setting up rain shelters in May 2023 to look at the ability of different grasslands to resist the impacts of drought. We have established rain shelters on a sub-set of the grassland restoration sites we have monitored in previous years.

Each rain shelter comprises a 3x3m metal frames and covered with sheet plastic which will act to totally exclude rain for a fixed period of time to simulate droughts. Each shelter has guttering attached to it so we can catch the run-off water and remove it from the plots. Our goal is to replicate these rain shelters both within sites (3 replicates), but also across sites (6 replicates) that themselves differ in their underlying complexity.
Setting up the shelters and transporting all the long metal poles to off-road field sites did prove to be difficult at times and included quite a few long walks from the van to the field site to get all the equipment where it needed to be. Who needs a gym?



L-R: Desperately trying to work out how to put it all together; Melanie carrying equipment out to the site; Getting stuck on the fields following an unexpected by torrential rain storm.
Once the rain shelters are up we will use them to simulate intense drought conditions for a 2 month period, with the goal of comparing the impact that this has on the plants, insects and microbial communities in subsequent years. We hope to understand how the decisions we make in terms of management during grassland restoration, including the choice and number of plant species established, will have long term consequences for the stability of these systems in a future where drought may be a more common yearly occurrence than it currently is.

April 2023: The weird and wonderful spiders of RestREco
By Andra Opris, Field Technician
With over 650 species of spiders in the UK occupying a variety of habitats, spiders represent a major invertebrate biodiversity group. They respond readily to small scale changes in environmental conditions, being fantastic indicators for ecosystem health and productivity. In wooded habitats, vegetation structure and complexity provide various micro-habitats necessary to fulfil their life histories. For that reason, evaluating spider assemblages is essential for our understanding of ecosystem function, and directly relevant in restoring natural woodland dynamics.

Paidiscura pallens, the Sputnik spider, is named after the egg sac’s resemblance to the first artificial Earth satellite. ©AJC1, 2019 (CC BY-SA 2.0; https://flic.kr/p/2ghKMTD);
As generalist predators, spider communities fulfil the ‘natural enemy’ role and regulate herbivore populations through top-down control. However, their different hunting strategies (or guilds) are reflected in functional differences in habitat use, including ground hunters, orb web-weavers, and ambush hunters. For example, vegetation dwelling spiders like long-jawed orb weavers (Family: Tetragnathidae) and comb-footed spiders (Family: Theridiidae) spin characteristic webs to capture prey while ground-dwelling spiders like wolf spiders (Family: Lycosidae) actively search for prey on the ground.


Theridion varians (Family Theridiidae) and Metellina segmentata (Family Tetragnathidae) are two of the most common spiders in Britain, reflected in high abundances in our samples. ©AJC1, 2019 (CC BY-SA 2.0; https://flic.kr/p/TMdiCS); ©AJC1, 2018 (CC BY-SA 2.0; https://flic.kr/p/2aUF5ur)
The RestREco woodland team collected woodland invertebrates by tray beating to maximise the sampling efficiency for all invertebrate groups we are especially interested in, including spiders. Additionally, many spiders were sampled in our Malaise traps (a modified tent with a collection pot to usually sample flying insects, such as hoverflies and bees) which will also be identified and included in our complexity analyses. Our arachnologist, Andra, has been undergoing our spider ID since December 2021 and explains the bizarre identification process:
Body form, coloration and eye arrangement are all useful characters when identifying spiders. However, identifying spiders to species requires assessing the morphology of their genitals. Male spiders don’t have penises. Instead, they use leg-like appendages called pedipalps to store and transfer sperm to females. To do this, the male first constructs a small web on which it deposits a drop of sperm. Then, he charges a specialised end of the palp, called the palpal bulb, with sperm via hydraulic pressures and delivers it to the female’s reproductive opening, called the epigyne, during mating. She stores the sperm in sperm chambers (spermathecae) until ready to lay eggs.


Episinus maculipes (Family Theridiidae), a once naturally scarce species in Britain, inhabits scrub and low canopies in woodland edges and spins a Y shaped web between vegetation © Andra Opris, 2022.
Both the palpal bulb and the epigyne have sclerotized (hardened) and rigid structures and only develop when the specimen reaches adulthood. The structure of these organs must fit together in a lock-and-key mechanism allowing mating, and vary greatly between species. Therefore, they provide a unique characteristic for identifying different spiders to their exact species.

A running crab spider, Philodromus albidus (Family Philodromidae) with distinctive epigyne structures. © Martin Cooper, 2015 (CC BY 2.0;https://flic.kr/p/yeRLRs)
Across species, male spiders have evolved an array of strategies to ensure reproduction success. Males of some species deploy copulatory plugs – disposing of the palpal bulb after copulation which remains anchored in the female’s epigyne, essentially inhibiting future reproductive attempts! Similarly, in some species the males actively avoid being eaten by the females, either by mating with freshly moulted (and thus vulnerable) females or offering ‘’nuptial gifts’’ – a wrapped up prey offering distracting the hungry female and prolonging mating.
Nonetheless, these interesting invertebrates play important roles in our project as part of our complexity and ecosystem function metrics. The data and results from RestREco will ultimately inform future restoration projects on how to restore complex and high functioning woodlands and grasslands, with diverse and resilient communities, including the weird and wonderful world of spiders!
March 2023: Conversations about conservation
By Prof. Rosie Hails, Director of Science and Nature, National Trust
The National Trust Advisory Group Conference was held on 27th February 2023 in Birmingham.
This is an important topic for the National Trust – now and always – because conservation of historic buildings, collections, archaeology, historic gardens and landscapes, as well as nature, lies at the very heart of its core purpose. This is also a discussion that is of direct relevance to RestREco, not just in how it is challenging our approach to ecological conservation, but also in considering conservation principles more broadly. For that reason, National Trust invited Jim Harris to speak at its annual Advisory Group Conference.
Although conservation has been part of the DNA of the National Trust since its formation 128 years ago, we live in unprecedented times – from climate crisis to biodiversity crisis; to energy crisis; to a crisis in community trust and listening. The world is changing, changing fast, and changing in ways that are unpredictable and difficult to model with any certainty. For this reason, the Trust brought together an interdisciplinary group of experts to challenge and discuss how conservation principles need to evolve to be fit for the 21st Century. We need to make deliberate choices in circumstances entirely different to when the Trust was founded – be that where to establish 20 million new trees, how our management of protected areas should change, or when and which collections’ objects should be repatriated to source communities.



Left: Jim Harris presents at the National Trust’s Advisory Group Conference [Photo by National Trust]. Top right: a chalk grassland rich in wildflowers [Photo by Maico Geert Weites]. Bottom right: Ancient woodland at the National Trust’s Slindon Estate, surveyed as part of RestREco fieldwork in May 2022 [Photo by Ross Barnett].
The UK is one of the most nature depleted places on earth and estimates suggest we are losing around two thousand species globally per year. Jim gave his perspective on how ecological conservation should change its emphasis and direction in ways that are fit for current and future challenges. Jim gave an excellent talk on ‘Restoring Forwards’ which embodied much of the ethos at the centre of RestREco. Emphasis was on restoring ecological processes to deliver functioning ecosystems fit for the future climate. He also challenged whether current policy for protected sites is sufficiently agile and flexible, given that the future distribution of species across the UK is uncertain. This is a hot topic, as protected areas such as Sites of Special Scientific Interest (SSSIs) have an important role to play in the future as ‘battery packs’ that have the potential to recharge surrounding landscapes with lost wildlife. What is abundantly clear is that we need to act urgently and at scale, not only enhancing the condition of protected areas and restoring priority habitats, but also in recreating the dynamic landscapes of previous eras. Only by acting at sufficient scale can we harness the power of ecological processes to restore ecological function.
In this sense conservation practice is about so much more than holding on to the past – whether object or habitat. It is future facing as we make active and considered choices about what to carry forward into the sustainable future we want to see.
February 2023: Uncovering our woodlands’ industrial past
By Anna Gee, former University of Stirling research technician
Over the past two summers, we have been out surveying 66 restored woodlands and 66 restored grasslands for ecological complexity and ecosystem function. While all our grasslands were former agricultural sites, our woodlands are on land that was previously either used for agriculture or industry. The industrial sites were previously coal mines, shale oilworks, or piles of waste material known as ‘bings’. Now, many are nature reserves, home to a diverse array of flora and fauna and providing accessible green spaces in the most unexpected of places.
The industrial revolution created huge demand for coal and oil. From the 1860s until the early 20th Century, Scotland was the world’s leading producer of oil, which was refined from shale. Huge amounts of waste shale were left over following extraction of the oil; six tons for every 10 barrels of oil. This waste was piled up into huge heaps, known locally as bings, that can still be seen from miles around.
The most iconic bings are the Five Sisters, near West Calder. We spotted the five pyramids rising sharply angled out of the flat landscape en route to survey the nearby Addiewell Bing Nature Reserve. This site was previously an oil-shale bing, though extraction ended in 1932. Over the next 50 years the abandoned site began to be reclaimed by nature, with early successional plants recolonising the bare soil. Reshaping and tree planting followed, and the site is now a Scottish Wildlife Trust reserve. It supports a range of habitats, and the rare moss species Buxbaumia aphylla, commonly known as bug-on-a-stick, has been recorded at the site for over 35 years.





Clockwise from top left: OS map showing Addiewell in the 1900s. [© Crown Copyright and Landmark Information Group Limited (2021). All rights reserved]; The Five Sisters [Source: westcalder.org]; RestREco field team at Addiewell Bing, June 2021 [Photo by Anna Gee]; Buxbaumia aphylla sporophytes [Source: indefenseofplants.com, photo by Bernd Haynold]; and Postcard, possibly from 1915, showing Addiewell Oil Works [Source: scottishshale.co.uk]
Not far down the road, in nearby Stoneyburn, is another RestREco survey site. Foulshiels Wood is now owned by project partners, The Woodland Trust, but as recently as the 1950s it was an active coal mine. Several of the paths at Foulshiels follow the routes of the old Wilsontown, Morningside and Coltness Railway, which opened in 1845 to serve the local collieries and oil-works. The mine was closed and capped in the 1950s and 60s, and in the 1980s a reclamation project began, with a mix of broadleaf and conifer trees planted. Today, Foulshiels is teeming with life, and many interesting plant species can be spotted. As you walk the quiet paths that criss-cross the woodland, surrounded by ornate orchids and delicate cottongrass, it’s strange to imagine that, not so long ago, carriages piled high with coal and shale used to travel those same routes.
We will be contributing to a Bio-Blitz at Foulshiels in summer 2023 with the Woodland Trust. Keep an eye on our twitter for us announcing this event!

OS map showing Foulshiels Wood in the 1950s. [© Crown Copyright and Landmark Information Group Limited (2021). All rights reserved]

Flora found in Foulshiels Wood. Clockwise from top left: Common wintergreen (Pyrola minor), Common Spotted-orchid (Dactylorhiza fuschii), Cottongrass (Eriophorum angustifolium – technically a sedge!), Field of cottongrass, Common Twayblade (Listera ovata), Columbine (Aquilegia vulgaris). [Photos by Anna Gee]
The last shale mine in Scotland closed in 1962, by which point it had become cheaper to import liquid oil from the Middle East. Many of Scotland’s coal mines also closed in the 1980s, despite local and national strike action. One such mine was Polmaise, near Stirling. Polmaise Pits 1 and 2 were sunk in 1904, and, after eight decades of mining, are now the site of a small woodland – another of our post-industrial woodland sites!
The importance of these mines for the community is clear, and its legacy lives on in institutions such as the Fallin Miner’s Welfare Society and Social Club and through the written and oral histories of those who worked in the mine. On the land itself though, steep slopes and large clumps of shale are now swamped and softened by ferns and willowherbs, as the industrial history of this site becomes gradually obscured by a tangle of new green shoots.

Left: Polmaise pits 1&2 in the 1940s. [© Crown Copyright and Landmark Information Group Limited (2021). All rights reserved] and right: Postcard of Polmaise colliery [Source: polmaiseproject.wixsite.com/ourminingheritage]

Flora growing on the site of the old Polmaise coal mine pits 1&2. Left to right: Hedge woundwort (Stachys sylvatica), Rosa spp., Pirri-pirri-bur (Acaena novae-zelandiae). [Photos by Anna Gee]
Photo credits detailed in captions.
January 2023: RestREco and Woodland Trust team up at BES 2022
By Dr Chris Nichols, The Woodland Trust’s Conservation Evidence Manager
Conferences are strange things. One minute you can be discussing with colleagues which flavour of hummus best sums you up. The next, you can be debating the relative merits of ecological complexity and its impact on resilience and nature recovery. Thankfully, the session I co-hosted with RestREco’s very own Kirsty Park at the 2022 British Ecological Society conference, focused on the latter. The title being: Is complexity important for ecosystem restoration? Spoiler alert. The answer is ‘yes’.
Conference confessions
It might be unwise to admit this in a blog aiming to publicise a group of presentations at a conference, but this year at the BES annual meeting, the talks came second. The real joy this time around was the space between the talks. Now that our working lives are dominated by virtual meetings, the opportunity to meet colleagues old and new in the flesh, often trumped the content of the presentations themselves.
That’s not to say that the talks aren’t important. Far from it. I was honestly inspired by the innovation and passion on display this year. The speakers in our thematic session were no exception, sparking thoughtful comments and debate in the closing panel discussion.
Back to the start
But to go back to the start, the inspiration for the session came from the questions posed by the Restoring Resilient Ecosystems project. Can we move restoration science forward by investigating complexity and resilience as aims in themselves? How do site factors influence complexity? How does complexity influence ecosystem function? How can we practitioners intervene to influence complexity?
These really important questions were covered by Emily Waddell in her talk during the session. Emily leads the woodland data collection and analysis part of RestREco. This overview of project plans and progress gave a tantalising glimpse into the project’s future impact. From the discussions had over coffee with attendees after, it felt that both Emily’s talk and the session itself did a great deal to drum up anticipation in the ecological community for fresh evidence to enhance conservation and restoration.
Using evidence
The role of my team at the Woodland Trust is to ensure our conservation activities are led by evidence. No small task for the UK’s largest woodland conservation charity which owns and manages over a thousand sites, and many more through our work with partners. Not to mention all the policy-influencing, campaigning and fundraising. Research and evidence are our bedrock.
Which is why the Woodland Trust and our Conservation Research Programme is delighted to be a project partner on the RestREco project. The opportunity to co-host a thematic session inspired by the project was the icing on the Christmas cake at this year’s, as always, wintery BES conference.



BES annual meeting at the Edinburgh International Conference Centre in December 2022 (left); the Woodland Trust stand and team at BES (top and bottom right)
#Content
Our keynote presentation from Holly Jones at Northern Illinois University set the stage with a global perspective on the need for ecological complexity to restore the world’s damaged ecosystems. Holly inspired us all with tales of bison recovery on prairies and birds flourishing once again on islands, all with a little help from encouraging more complex and diverse ecosystems.
Do you the know the difference between restoration and rewilding? And how both approaches can be used in a complementary way to achieve greater complexity and restoration outcomes? Nathalie Pettorelli of the Zoological Society of London described this continuum, followed by the Woodland Trust’s Chris Reid, who gave a practical perspective on how to put this evidence into action. Chris took us through the new Woodland Creation Guide and associated Tree Species Handbook, which encourages structural complexity from the outset in woodland creation and restoration projects.
There are many objectives that a woodland creation project might want to achieve. Felicity Monger’s talk explored how flood reduction objectives can be enhanced by ecological complexity. Felicity’s presentation featured research involving the Woodland Trust’s new site, Snaizeholme, in the Yorkshire Dales National Park, which is fast becoming a major research hub for the Trust thanks to a flourishing formal collaboration with University of Leeds.
Ambitions to restore resilient ecosystems are all very well, but if we don’t have the right funding mechanisms in place, achieving those goals will be impossible. Sophus zu Ermgassen of the University of Oxford presented insight into how we scale up funding for ecosystem restoration, arguing that relying on private sector funding is a risky proposition. Sophus is working on a big European-funded project investigating how we upscale forest restoration (‘SUPERB’).





Clockwise from top left: Panel discussion at end of thematic session on ‘Is complexity important for ecosystem restoration?‘; Sophus zu Ermgassen presenting on ‘scaling up funding for forest restoration’; Felicity discussing the benefits of woodland restoration for flood reduction; Emily introducing the RestREco project; and Nathalie explaining how rewilding and restoration can be used in a complementary way to achieve restoration outcomes.
Complexity need not be complicated
As RestREco colleagues said in their 2022 Ecography paper: definitions of complexity need not be complicated. Our thematic session showed that despite this, achieving restoration outcomes is not always straight-forward. However, there is hope, and the Woodland Trust will continue to fund and collaborate on ground-breaking research projects such as RestREco.
For some further reading, check out the Woodland Trust’s latest issue of Wood Wise, our science and practice magazine, on nature recovery at scale, and the special edition of the IES Environmental Scientist journal on ecosystem restoration.
In case you were wondering, if I were a hummus, I’d be smoked hummus. Under-appreciated, pretentious, and of course, oh so complex.
Photo credits: Ross Barnett and Chris Nicols
December 2022: Roundup of the year!
By Ross Barnett and Dr Emily Waddell, woodland folk
January
We saw out out the cold winter months in the warm labs of Stirling and UKCEH, identifying our thousands of invertebrate samples! At Stirling, we focused on first choosing our target orders for our tree beating samples, which included beetles, true bugs, earwigs, lacewings, spiders, and harvestmen. These target groups fill a range of trophic roles and will allow us to build inferred foodwebs for each of the sampled trees within woodlands. Food web complexity can then be added into our wider considerations of ecosystem complexity. At UKCEH, research technician and entomologist, Maico Geert Weites was identifying similar target groups from the grassland suction samples (for a great summary see here).



February
This month, the woodland team started preparations for the 2022 field season by finalising the site selection of our natural experiment. Matt and Emily trawled through thousands of potential woodlands in the National Forest Inventory (NFI) database to find the remaining 30 woodlands in England. Each potential woodland was cross referenced with Google Earth imagery and historic land-use maps, to find woodlands with the right combination of age, former land use (agricultural or industrial) and area of woodland in the surrounding landscape. After some detective work to find the landowners and gather permission to survey sites, we had the final 30 sites we needed for ecosystem complexity surveys.
In both habitats, we also selected the 18 woodlands and 18 grasslands for our detailed surveys measuring ecological function. These sites were selected to represent a gradient of complexity, using proxy measurements of complexity. For grasslands this was plant species richness and for woodlands, the standard deviation of tree diameter, which has been shown to be important in other woodland creation sites (see the WrEN project here).
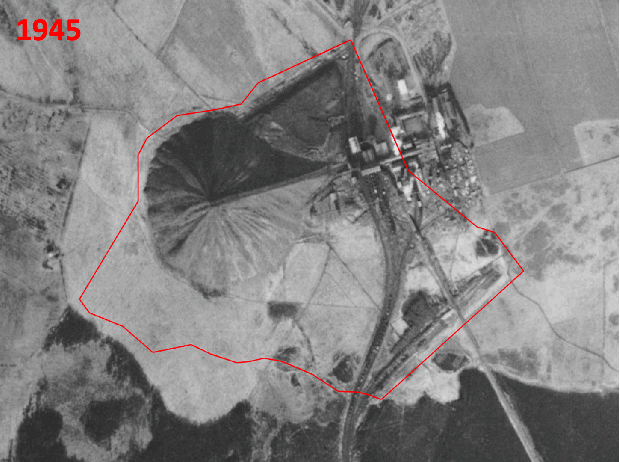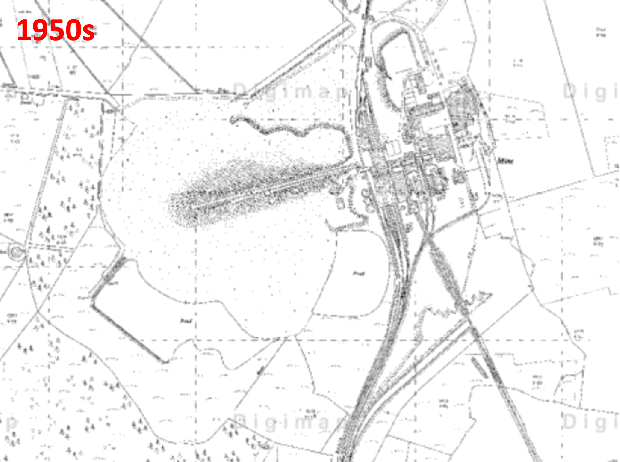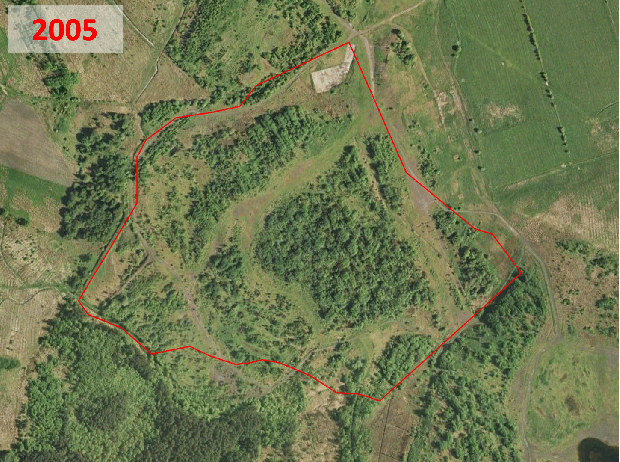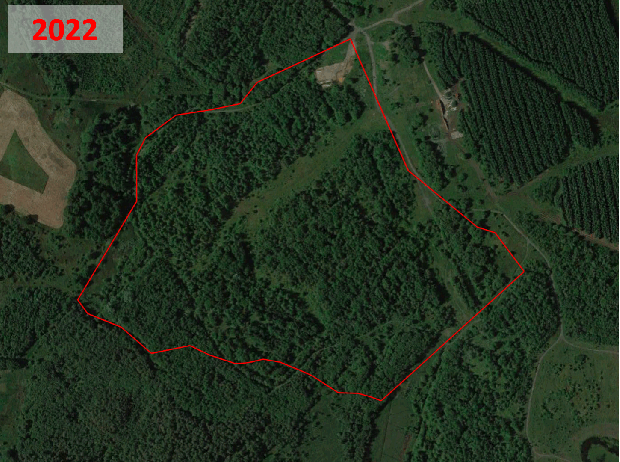
Historic aerial photography, maps and satellite imaging showing a RestREco woodland site – image of a mining site in 1945, confirmed in OS Maps from 1950; OS Maps from the 1980s show the spoil heap; with established trees in Google Earth images by 2005; woodland in 2022 satellite image. From this we can determine that the site was planted in the 1990s, and is circa 30 years old.
March
Down at Cranfield University, postdoctoral researcher Oscar Aguinaga Vargas and colleagues were busy designing the best DNA extraction procedure for over 600 soil samples, across all 66 woodlands and 66 grasslands! This procedure needed a bit more thought due to the quantities of chalk within some calcareous grassland samples, as chalk reacts with certain buffers making extractions tricky. After some discussion, Oscar and colleagues figured out a plan of action and started with extractions. Find out more about Oscar’s labwork in his blog post from August below.
The grassland team spent March setting up intervention plots at 10 of our restored grassland sites. At these sites, we will simulate common management strategies for calcareous grasslands, such as seeding and carbon addition. Each site had four 5x5m plots: seeding only, carbon addition only, seeding plus carbon addition and a non-intervention control plot. Carbon was added through a mixture of wood shavings, starch and sugar, and the seeding mixture contained native UK wildflowers. We will monitor the effects of these management interventions to determine what effect they have on the development of complexity.






Top row: a selection of soil samples in the field and Oscar processing samples in the lab (right). Bottom row: Maico setting up the management interventions in grasslands
April
This month we deployed the AudioMoth recorders for spring acoustic surveys to record the peak of bird singing activity. Between the woodland and the grassland team, we deployed 100 AudioMoths, recording over 14,000 minutes of acoustics, which are currently being analysed for acoustic indices. Acoustic indices are a relatively new tool in ecology. There are a range of different indices, each working in slightly different ways, but in general, they are means of quantifying the diversity of sounds within an audio file. These indices have been shown to correlate with variables such as bird diversity and habitat disturbance. We look forward to seeing how they reflect complexity in our woodlands and grasslands.



Ross deploying AudioMoths in woodland sites (left), an AudioMoth in a woodland (centre) and grassland (right)
May
This month marked the beginning of a summer of fieldwork in the grasslands and woodlands, starting ecosystem function measurements in both habitats and finishing off the complexity surveys in our woodlands.
The woodland team made their way to Sussex to visit two of our woodland “wildcard” sites, Knepp Wildland and the National Trust’s Slindon Estate. Our wildcard sites are a mixture of ancient woodlands, ancient grasslands and rewilding sites that we are measuring the same ecological complexity metrics in. We will see how these potentially very different wildcard sites compare in terms of complexity to our much younger and more traditionally restored woodland and grassland sites.
Rewilding projects typically involve leaving nature to recover without intervention, and Knepp Wildland is one of Britain’s largest rewilding project. It was also our only site to be visited by both the woodland and grassland teams, due to the heterogeneity of the landscape created by the mix of introduced large herbivores, which act as ecosystem engineers. Having read so much about the rewilding at Knepp, it was fantastic to be able to visit and spot the nightingales, stags and storks for ourselves!
We were joined for our woodland fieldwork in England by Cecilia De Sanctis,a master’s student from Sapienza University of Rome, who visited us for the whole summer on a research scholarship. Cecilia is also an illustrator and produced a fantastic cartoon of the English woodland team after her time working with us! See more of Cecilia’s work here.




Left hand side: the field team carrying out a range of surveys at Knepp Estate and Slindon Estate and visiting a viewing platform at Knepp (bottom). Right hand side: Cecilia’s illustration of the team – spot who is who!
June
Over the summer, we returned to a subset of sites (18 woodlands and 18 grasslands) to carry out ecosystem function measurements. We did this to test how ecosystem function changes along a gradient of complexity, with our prediction that highly complex ecosystems with many species and species interaction, will support more functions.
To determine predation rates, we deployed artificial caterpillars made of plasticine (we swear it’s real science!). The soft plasticine takes an impression of any bite marks left by predators like birds, mice, and beetles. In woodlands, herbivory rates were determined by sampling branches of birch and oak, and measuring the extent of insect herbivory on the leaves. The grassland team measured herbivory in Plantago and Raunculus (buttercup) plants. In grasslands, Maico was also busy setting up grazer exclusion cages to quantify primary productivity. These small wire cages prevent sheep, cattle, and deer from being able to eat the plants, and so that we measure plant biomass and calculate growth rates.
We also visited two more “wildcard” sites – both ancient woodlands this month. One site is intersected by the famous West Highland Way, and represents a large piece of globally rare Celtic Rainforest – oak forests characterised by high humidity, stable temperatures, and, of course, high rainfall.





Top row: Plasticine caterpillar in grassland (left) and with bird predation marks (right). Bottom row: Herbivory measurements on oak (left), grazer exclusion cage for grassland biomass measurements (centre), Mudgock wood, an Ancient woodland in Scotland (right).
July
This month we continued with our ecosystem function measurements with the woodland fieldwork assisted by a team of Stirling University undergraduate students (thanks everyone!). To measure pollination rates, we used organza bags, to exclude pollinators from one flower bud with another bud open to be pollinated. Once mature, we harvested both bagged and unbagged fruits and counted any seeds.
We collected spiders and predatory beetles in each woodland, which have been sent for DNA metabarcoding of their gut content analysis to assess the diversity of diet and build food webs. This was a particularly enjoyable part of fieldwork, lifting rocks and peeling bark from deadwood to find as many ground beetles as possible! Unfortunately, this element of the work had to be postponed in the grasslands due to the impact of the drought, which decimated the plant and invertebrate community (See our September blog by UKCEH’s Ben Woodcock).





Top row: A bagged buttercup with pollinators excluded (left), and an unbagged buttercup being visited by a pollinating thick-thighed beetle (Oedemera nobilis, right). Bottom row: ground beetles (Carabidae) collected for gut content analysis (left), the impact of the drought on one grassland (centre) and satellite images showing the extent of the drought across the UK (right).
August
The woodland team finished fieldwork on complexity metrics in our 30 English woodland creation sites and six ‘wildcard’, with a final round of AudioMoth deployments targeting bats, malaise trapping for flying insects and soil sampling. This means all field data on ecological complexity is complete, and analyses can start!
At our 36 ecosystem function sites, to measure parasitism rates we collected seed heads from thistles (Cirsium palustre & Cirsium arvense) in woodlands and Centaurea nigra and Lotus corniculatus in grasslands. The flowers of these plant species are commonly used by larvae of fruit flies as a food source, but these larvae can in turn be parasitised by wasps. We are rearing these seed heads to see what emerges, a fruit fly or parasitic wasp!
At Cranfield, Oscar performed physicochemical characterisation of all the soil samples, which includes measurement of pH, conductivity, texture, total nitrogen and total and organic carbon. Samples were also dried, grounded, and preserved as soil archives for any possible further analysis in the future.



Malaise traps and AudioMoths in the field (left & centre), and Undergraduates Georgia and Will conducting soil sampling (left).
September
We wrapped up the last of the ecosystem functions woodland fieldwork with soil sampling for macrofauna. We borrowed a very cool corer from colleague Frank Ashwood at Forest Research, which fits a plastic tube inside the coring mechanism, so we can extract an undisturbed sample with soil layers intact. We then extract the soil invertebrates in Tullgren funnels for identification. The grassland team also used the same method at their 18 ecosystem function sites luckily earlier in the year before the drought hit!
September saw Sam Rogerson, technician and entomologist extraordinaire leave the RestREco Stirling team to start an exciting PhD on insect colonisation of secondary woodlands with the WrEN project. We wish Sam all the best with this and all future endeavours! Andra Opris, our technician with a special spot for all things arachnid then joined us full time to help with our invertebrate identification. Welcome to the team, Andra!



Soil corer & core for sampling soil macrofauna (left), Ross at Forest Research extracting the soil invertebrates using Tullgren funnels (centre), and new woodland team member Andra Opris (right).
Emily presented an introduction to RestREco at the INTECOL conference, in a session titled ‘Restoration and Rewilding: upscaling activities to reverse global terrestrial ecosystem degradation’, chaired by RestREco’s James Bullock alongside Nathalie Pettorelli from ZSL. Read more about this session and watch a recording of the talk in our October blog post!

Slide from Emily’s INTECOL presentation illustrating the link between complexity, functionality and resilience.
October
Some of the woodland team travelled to our English woodlands to do some preparatory fieldwork for our management interventions experiments. At ten sites part of or close to the National Forest, we set up three plots: a control plot, a thinning only plot and a thinning plot with the thinned wood left in situ. We are aiming to remove 30% of the basal area within each thinned plot to open up parts of the canopy and increase woodland structural complexity. You can find out more about this in Matt’s November blog.
At UKCEH, Maico and Ben identified all the woodland spider and beetle gut contents samples as we want to conduct this analysis on the same species at all sites if possible. Oscar managed to complete all the soil DNA extractions, with very good quality for sequencing! More on how this DNA analysis will be used can be found Oscar’s blog from August.
RestREco project collaborated on a special issue of IES‘s environmental SCIENTIST journal on restoration ecology. The articles within were written by consortium members and project partners on a wide range of topics including challenges of setting & achieving goals in restoration, policy frameworks, restoring for ecosystem complexity and lots of case studies. Download the full issue here.



Trees marked for thinning (left), the team in the field (centre) and the front cover of the environmental Scientist (right).
November
Following our preparation work in October, the intervention work began in November with Chris Eckton of Forestry England completing thinning at three of the ten intervention sites.
Led by Emily, the woodland team published an article in the autumn issue of Wood Wise, the Woodland Trust’s journal. This issue focuses on nature recovery at scale, and Emily introduced the rationale behind RestREco and discussed the plans we have for data collection and synthesis.
At Cranfield, Oscar started processing the samples for our 36 ecosystem function sites. Procedures for analysis of respirometry, microbial biomass and potential nitrification have been designed, with analysis planned to begin next year. This data will give us insights about the microbial mediated soil functions at different stages of restoration.
At the end of the month, we had a full-day online meeting to discuss all the data we have collected on biodiversity and complexity, and various approaches to analysing these data. In this meeting, we were also introduced to bioinformatician, Dr Alexey Larionov, who is based at Cranfield and will be joining the consortium to assist with complexity analyses. Welcome to the team, Alexey!



Plot with trees thinned and deadwood removed (top left) and plot with trees thinned and deadwood left (bottom left), and front cover of the Wood Wise issue (right)
December
For both woodlands and grasslands, December has been dominated by processing invertebrate samples and working on identification and preservation. We have also sent updates on our progress to all our woodland landowners to keep them informed about the project.
Rosie Hails gave a presentation to the People’s Assembly for Nature on the 4th of December. The presentation was titled “What do we mean by nature restoration?” and mentioned the work being done by RestREco.
Some of the team attended the British Ecological Society Annual Meeting in Edinburgh this week – a fantastic chance to meet people and chat about restoration, especially in the Thematic Session titled “Is complexity important for restoration?” and discussion panel chaired by Kirsty Park alongside Chris Nicholls of the Woodland Trust. Emily presented a talk in this session titled “Restoring Resilient Ecosystems – Future restoration should enhance ecological complexity and emergent properties”, in which she provided an excellent summary of the basis of the RestREco project.



Left hand side: Emily presenting RestREco at the BES Annual Meeting (top), the panel discussion (bottom). Right hand side: A copy of our update for landowners and stakeholders.
As you can see, it’s been a very busy year for all of team RestREco! We are going to take a well-earned breakover Christmas and New Year, and we’ll be back with more updates in 2023 😊
November 2022: Can management interventions speed up the development of ecosystem complexity?
By Dr Matt Guy, Spatial scientist at Forest Research
In the UK, habitats have been manipulated by humans for resources for centuries and many native species are now dependent on the conditions this creates. Woodlands and forests have historically been managed to provide wood for fuel and building materials and many of the techniques, such as thinning and coppicing are still used in commercial forestry and conservation. As part of the RestReco project we are interested in how some of these techniques can be used to enhance development of site level complexity, specifically thinning and deadwood management.
Thinning
Thinning, removing select trees from a stand, is a standard practice in new woodlands. Traditionally, tree planting in woodland creation sites occurs at relatively high densities; 3m spacing is the minimum but 2m is often used. This density encourages fast, straight growth and rapid canopy closure. After around 10-15 years, resources such as light and water become limiting and tree growth will slow or stop altogether. At this stage the stand will be thinned to give the remaining trees a greater share of the available resources and promote healthy woodland development.
Thinning can occur at random (e.g. every 4th tree removed) or the tree removal can be targeted to manipulate the trajectory of woodland development. In commercial forestry thinning is often used to increase the overall stand quality by removing undesirable trees or competitors from around the best individuals or create even aged stands by removing individuals from the lower canopy. However, thinning can also be used to increase woodland structural diversity by removing individuals from the upper canopy (crown thinning), opening the stand, and encouraging growth of trees under the canopy.
New woodlands are often fairly structurally homogenous so targeted thinning has the potential to increase the structural diversity resulting in a greater array of niches for species, increasing site complexity.


A young woodland creation site near Glasgow showing densely planted trees which may benefit from thinning (left); canopy of a more open 40-year old woodland creation site (right)
Deadwood
Deadwood is a vital component of a woodland ecosystem; up to 20% woodland species are dependent on it for part of their lifecycle. Typically, these deadwood habitats develop over time as a woodland ages, trees and tree branches are lost creating standing and fallen deadwood, the stumps and roots of dead trees start to decay and eventually in ancient trees the heartwood starts to decay and hollow out.
Deadwood habitats in new woodlands are often scarce as most of the young trees are still alive and growing. This is often further compounded by a tendency for people to remove deadwood, through fear of spreading disease, to maintain a tidy woodland or for safety. Adding recently felled trees to new woodlands may speed up the creation of deadwood habitats, aiding the support of deadwood dependent species and increase ecosystem complexity.





Clockwise from top left: Jelly ear fungus (Auricularia auricula-judae) growing on a dead fallen branch, with Ross and Sam measuring deadwood in background; a rove beetle (Othius punctulatus) found inside deadwood; ‘Dead man’s fingers’ fungus (Xylaria polymorpha) growing on deadwood in a Scottish Ancient woodland; Snail-eating ground beetle (Cychrus caraboides) in deadwood; Emily and Ross measuring a large piece of deadwood in a Scottish Ancient woodland.
Experimental set up
To test if thinning and deadwood additions can increase ecosystem complexity in new woodlands, we established a Before After Control Intervention (BACI) experiment across 10 sites. BACI experiments are a “gold standard”, but often hard to establish and implement in ecological studies. As the name suggests they require an assessment of baseline conditions, an intervention and an assessment of the conditions after this intervention along with a control, where no intervention has taken place.
To ensure we had baseline conditions, the 10 sites were selected from the wider suite of 60 sites that were surveyed over the past two years to link complexity with age and landscape context. At each site, we then used this survey data to identify 3 plots (of the 5) that were structurally similar. Each plot was then assigned one of three treatments: Control, Thinning and deadwood addition and Thinning only (all deadwood removed from the plot). The treatments were largely assigned to a plot at random, although occasionally site conditions or landowner requests dictated this. Figure 1 shows the experimental set up at Coton wood, a Woodland Trust site.
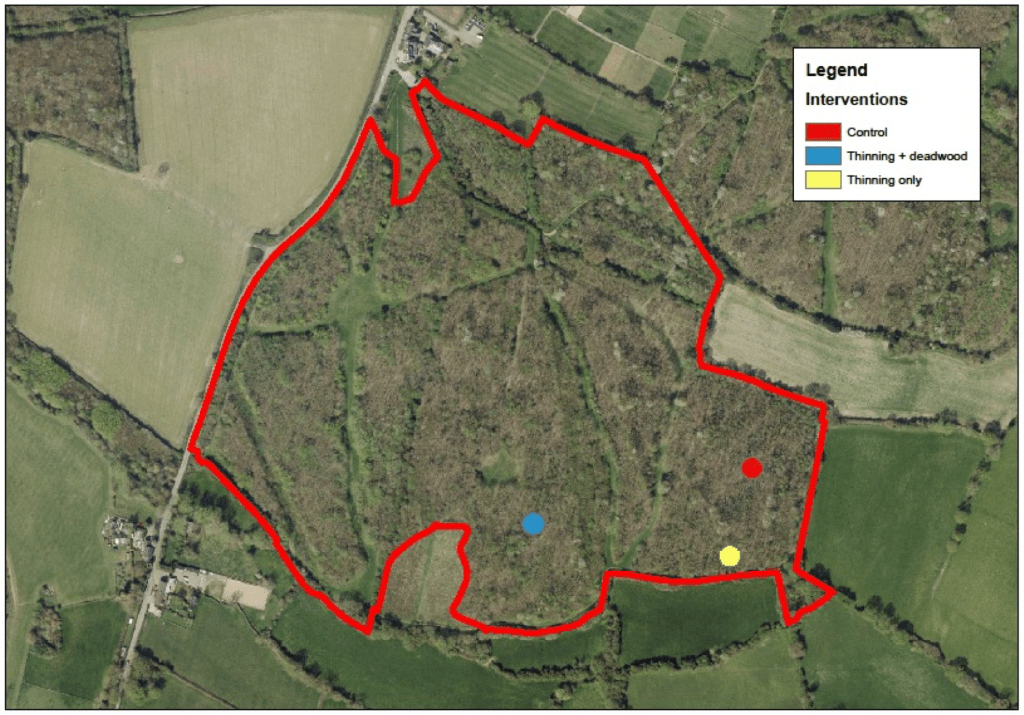
Figure 1. Siting of the three plots and the associated treatments at Coton Wood, a Woodland Trust site.
Management interventions
One of the biggest advantages about a BACI experiment is we have a record of the starting conditions and a control to compare any changes to. Therefore, provided the same relative treatment is applied across the sites inter-site differences in baseline conditions are less important.
We designed the Thinning treatment to reduce the basal area (the total approximate cross-sectional area of each tree measured at breast height) by 30% and that the Deadwood additions will comprise all trees felled by thinning. This makes the Thinning treatment consistent across sites but the number of trees felled will vary with initial conditions. In addition, it makes the dead wood additions consistent as the resulting deadwood:live wood ratio in the Thinning plus deadwood treatment is the same across all plots (30%).
As noted above the size and type of tree removed can have differing effects on woodland structure and development. The aim of the management interventions is to try and increase ecosystem complexity. Previous work has identified woodland species diversity is associated with the variation in tree size, likely linked to increases in species niches/microhabitats. Here we designed a thinning strategy that would reduce the overall basal area by 30% and increase the variation of tree size. Using the base line data the trees were separated into size classes based on the measured trunk diameter measured at breast height (DBH). The number of trees to be removed from each size class was calculated relative to the plot level mean and standard deviation of DBH; the smaller the standard deviation of the DBH, the more trees are removed from size classes around the mean DBH, whereas, if a plot has a high DBH, individuals from a larger number of size classes will be removed. Figure 2 provides before and after measurements for each treatment at Coton wood (the map in Figure 1), how many trees to remove from each size class and the effects on basal area and standard deviation of DBH.
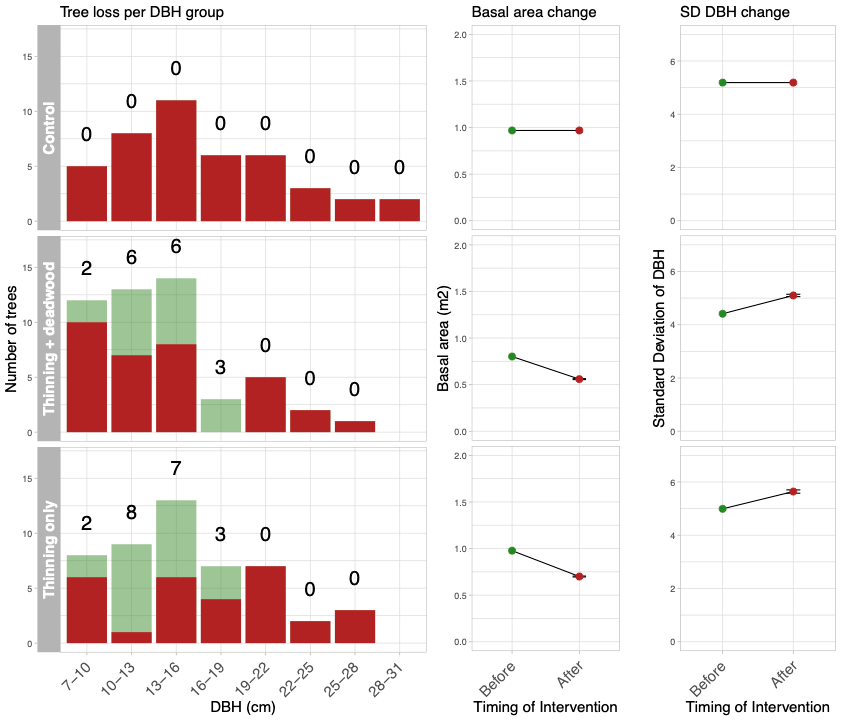
Figure 2. Three panels showing the trees removed from each size class during the thinning interventions at Coton Wood and the effects on the plot basal area and standard deviation of the DBH (Diameter at Breast Height (cm)). In all panels the green bars and points show the baseline conditions and the red bars and points indicate the conditions after the thinning. The numbers above each bar in the left-hand panel indicate the number of trees to be removed from the DBH size class.
Carrying out the management interventions is not a small job. As shown in Figure 2. there are as many as 20 trees to be felled (and removed) and these are up 19cm diameter. As with a lot of ecological research, this work would not be possible without the kind work of site managers, land owners and voluntary groups. Here we worked with ecologists at Forestry England, Woodland Trust contractors and a volunteer conservation group, Hartwood, who offered their expertise, experience and time to complete the work in line with best practice guidelines.
Using the calculations outlined above, we identified how many trees in each size class needed to be removed from plots across the 10 sites. Then three of the RestREco team met with the different groups carrying out the work to assign plots to specific treatments and markup trees to be felled. See below for photos and videos of our ‘tree marking’ fieldwork! The thinning and deadwood additions are taking place right now and should be complete by the end of January.






Photos from our intervention preparation trip showing the marking of selected trees to be felled and Kevin, Emily & Matt enjoying the variable weather (top four photos and bottom left). Management interventions showing the two treatments thinning and deadwood removal (right centre) and thinning with deadwood left onsite (right bottom).
Assessing plots for change
The final part of a BACI experiment is to re-survey plots after the intervention has had time to take effect. The intervention plots will be re-surveyed for a variety of complexity measures in 2 years’ time. This is a relatively short time for ecological responses in the system (timing of this is largely driven by funding restrictions), but we are anticipating that some measures to be influenced by the management. For example, it is anticipated that ground flora and soil measurements may respond reasonably quickly to the canopy opening up, whereas we are not likely to identify significant increases in saproxylic invertebrates as deadwood take time to decay. We may also identify secondary, short term benefits of deadwood addition, such as protecting emerging vegetation and seedlings from deer browsing pressure, which in turn may increase complexity. However, due to strong links with land owners and partner organisations, such as the Woodland Trust and the National Forest Company, we are hoping that some of these experimental plots will provide a basis for future research and monitoring.
Conclusion
Habitat management and manipulation has occurred for centuries and some techniques are well established and used regularly in commercial forestry as well as conservation. Here we are testing if these techniques can be used as a tool to speed up the development of ecosystem complexity in woodland creation sites. If successful, we hope that the result of these projects can be used by land owners, managers and policy makers to aid the creation of complex and resilient ecosystems.
Photo credits: Emily Waddell, Sam Rogerson, Ross Barnett, Cecilia De Sanctis, Kevin Watts and Chris Eckton
October 2022: Restoration and Rewilding at INTECOL 2022
By Dr Emily Waddell, Woodland postdoctoral researcher
INTECOL 2022 was my first time presenting at an international conference as COVID-19 hit in my final year of my PhD and scuppered my plans to attend the Association for Tropical Biology and Conservation conference in Colombia. I was excited to have my talk accepted under the session ‘Restoration and Rewilding: upscaling activities to reverse global terrestrial ecosystem degradation’, moderated by RestREco’s Prof James Bullock (UKCEH) and Dr Nathalie Pettorelli from ZSL’s Institute of Zoology. As we were still in the middle of our field season, attending in person was going to be tricky, but luckily the conference was hybrid, allowing myself and others to participate online from home.
There were an interesting mix of talks and topics across the week, from the usual ‘impacts of climate change’, ‘land-use and biodiversity’, and ‘utilising remote sensing tools’ to more unusual but thought-provoking topics such as ‘Decolonizing science and fieldwork’. I enjoyed watching excellent plenaries each day, including ‘The ecological and social shaping of functional diversity’ and ‘Sensory pollution in urban environments’, which featured some great audio and animations! There were always several sessions running in parallel, which meant it was difficult to choose which to watch live, but the perk of hybrid conferences is the ability to access recordings afterwards (see below for mine!).
The ‘Restoration and Rewilding’ session had a wide range of speakers from restoration projects across the globe, including ‘Active vs. Passive restoration in tropical South America’, ‘Restoring wildlife corridors in East Africa’ and the ‘Rewilding of the Chernobyl Exclusion Zone’. I particularly liked the discussions around defining ‘rewilding’ and ‘restoration’ (as these terms are often used interchangeably), and how these two potentially complementary approaches may be used together to meet common goals (including a talk by moderator Nathalie Pettorelli). Also, the importance of ‘not just having the parts present, but that the parts need to work together’. For example, you can’t plant tree seedlings and expect that a fully functioning forest with all the ecological processes and functions will develop! This is something which is at the heart of the rationale behind the RestREco project, that restoration projects should aim to restore complex, high functioning ecosystems, which are resilient to future environmental change.
At the end of the session there was time allocated to a wider discussion between moderators, speakers, and the audience. One of the topics discussed was if disturbed areas are left with no active interventions by humans, will non-native (and potentially invasive) species just take over, and if so, is this a problem that needs to be actively managed, or is this just part of ‘rewilding’ that we need to accept. As someone who did their PhD on invasive species (see here if you are interested!), it is a topic I found particularly interesting and in my opinion the answer is both….As the planet becomes more and more connected, and the environment is changing, it is inevitable that non-native species will spread faster than we can control. Also, if some of these non-natives are able to adapt to the changing climate better than similar native species, then they may become an important part of future restored species communities, especially if they can restore functions or processes that have been lost from the ecosystem. In addition, most non-native species pose little harm to the native community with only 10-15% considered ‘invasive’, meaning they cause ecological and/or economic harm. Thus, when restoring ecosystems, I think we should strive to control highly invasive non-native species that could dominate the recovering species community, both known invasives and any newly introduced species that have the potential to become invasive (identified through risk assessments based on species’ biology, geographic origin, and pest status elsewhere).
This conference and session gave me lots of food for thought as I begin to think about analyses and write up of the RestREco data we’ve collected so far, and the plans for our management intervention experiment.
Click on the video below to hear a recording of my talk!
September 2022: Crispy chalk grasslands and withered woodlands – the impact of summer’s drought
By Dr Ben Woodcock, Ecological Entomologist at UK Centre for Ecology & Hydrology
Fieldwork is in full swing across the chalk grasslands in southern England. As stunning as this area can be under normal conditions, the recent drought has had a profound impact. July 2022 saw record temperatures and with much of southern England receiving only around 20% of the rain of an average July. Some areas in southern England received even less than 10% of the average rainfall. This has wreaked havoc on these grasslands, leaving them arid and brown with much of the normally all too evident insect life absent. The browning of the grasslands has been so widespread that it can easily be seen on satellite imagery.
SENTINEL-3 satellite image from August of Great Britain showing the intensity of the summer drought (Photo credit: @spatialanalysis/Twitter)
This has caused some issues for our surveys and we have had to postpone some elements of the fieldwork for now. It is a struggle to do pollinator surveys if there are no plants in flower and measuring water infiltration rates on bone dry soil is also far from ideal. Moreover the dry weather has caused issues for farmers. Grasslands that were not originally going to be grazed had sheep moved into them as the other fields did not have much to offer the sheep anymore!



A grassland field site in Oxfordshire turned brown with barely any green left and sheep grazing in distance (left); one of our Wiltshire grassland sites on 16th June 2022 (top right) and the same field on 11th August 2022 (bottom right)
While it would be nice to say these temperatures are unprecedented, this is another in a string of years where what may have once been considered unheard of temperatures are reached on a regular basis. What will be the long term impact for the communities of plants, insects, birds, mammals and microbes that inhabit these grasslands? This is true not just for these areas where restoration is being attempted, but also for the historic species-rich chalk grasslands (e.g. Salisbury Plain) which have typically formed the target communities for such restoration projects. It is highly likely that what we currently perceive as ‘species-rich chalk grassland’ represents a transient community that is the product of relatively recent climatic and management conditions, and one that may have been compositionally quite different in even the recent past. For example, during the Medieval Warm Period (somewhere between 1000-1200) or the Little Ice Age (1400-1800). While this is undeniably a beautiful and diverse community, both its long term persistence under climate change and its historical basis for use as a reference community to which restoration should aspire needs to be questioned. Indeed, this is one of the core aspirations of the RestREco project. To try and understand how restoration (both for grasslands and woodlands) can be developed in the future in a way that moves beyond this paradigm of simply trying to replicate idealised historic communities, and by doing this addressing the need to ensure that these restored habitats are robust to the ongoing perturbations that will threaten them in the coming years and decades. While this does not necessarily mean that many of the species we consider typical of calcareous grasslands may not continue to be a mainstead of restored grasslands in the future, it does introduce flexibility so that the focus may move away from simply species composition, to the capacity of these systems to remain resilient in the ecosystem processes that they underpin.



Walk into woodland site near Market Bosworth showing dried out grassland (left); Sycamore tree with leaves turning brown in August (middle) and ‘false Autumn’ at a woodland site near Northampton on 15th August 2022 (right)
In particular, it is the underlying complexity of the trophic interactions, across a range of taxonomic, spatial and temporal scales that may be the key to delivering restored grasslands robust to environmental change. This focus away from traditional endpoints for grasslands restoration (e.g. species-rich chalk grasslands) gives a new flexibility that provides avenues of opportunity to future-proof our world to change. The ever more obvious impacts of climate change make this new focus vital for the future stability of the environment.
Photo credits: Maico Geert Weites and Ross Barnett
August 2022: Analysing soil microbial complexity
By Dr Oscar Aguinaga Vargas, Soil postdoctoral researcher
One of the components of RestREco is the analysis of the soil microbiology of different restoration sites to determine soils complexity and to incorporate this new knowledge into ecosystem restoration. Soils are full of life, they contain an enormous diversity of living beings, including microorganisms (e.g., bacteria, archaea, fungi, protozoa), nematodes, collembola, mites, earthworms, termites, ants, among other forms of life. This vast biodiversity is mainly due to the heterogeneity of soils. In one landscape, we can find soils with different physical (e.g., texture, structure, porosity, permeability) and chemical (e.g., pH, salinity, nutrient content, organic matter) characteristics. Therefore, these different soil environments will create diverse habitats that can hold distinct forms of life. These variations also occur at the microscopic level (soil microhabitats). For example, oxygen availability in soils will determine the formation of microhabitats for aerobic, facultative, and anaerobic bacteria, all of them with different survival strategies (depending on whether they can use oxygen or other chemical compounds for energy production). These changes can be observed within a tiny scale of soil depth as oxygen concentrations can drastically change withing a few millimetres. Soil microorganisms are also connected to each other through different interactions. For example, microbes with a vast inventory of enzymes will breakdown complex food sources into more simple nutrients that allow specialized bugs to thrive. Following the oxygen example, those bacteria capable of using oxygen for energy production will modifying the chemistry of other compounds, making them suitable nutrients for other species with key roles such as sulphur and nitrogen cycling. In RestREco, we understand microbial soil complexity as the number of different microorganisms living in it, and the connections between them that are occurring in the different soil microhabitats.
Soil degradation is the decrease of soil chemical, physical and biological quality. This can lead to the loss of ecosystem services such as food production, water purification, carbon storage and climate regulation. Efforts to recover soil quality are focused on aboveground restoration, mainly revegetation and reforestation, with the aim to stabilize soil and provide organic matter. However, a key factor for successful soil restoration is located underground. The recovery of soil microbial communities, their diversity, and interactions are also necessary.
Thanks to our colleagues in the field, we have received several soil samples from grasslands and woodlands over the past months. The samples come from sites with distinct states (e.g. post-agricultural and post-industrial lands) and characteristics of restoration. We have designed and optimized a method for extracting DNA from all these samples. Over the past weeks, we have extracted DNA from all the organisms that inhabit or somehow left this molecular fingerprint on the soils; but for now, we will only focus on the microbial DNA (archaea, bacteria and fungi).






Clockwise from top left: Undergraduate students Georgia and Will collecting soil samples; Sam, Ross and Emily sampling soil (‘mud’) in the rain; Measuring out the top 10cm from a soil sample in a Scottish post-agricultural woodland; Clay soil in a post-agricultural site; Two soil samples from post-industrial woodland sites, showing pieces of brick and coal.
We are expecting between 1 – 3 million microbial DNA sequences per samples that we will analyse through DNA metabarcoding. This approach will help us determine the taxonomy and abundance of the microbiome of each sample. The results obtained will allow us to elucidate the taxonomic structure of the soil microbiomes (necessary to understand its complexity). Moreover, we will be able to correlate the taxonomy with environmental factors (chemical and physical soil properties) and with soil functions (litter degradation, nitrogen fixation) to determine whether these variables influence taxonomic assemblages.


Oscar in the lab carrying out DNA extractions (left) and sieved, grounded and dried soil samples ready for nutrient analysis (right)
The next information we need to measure complexity is the connection between the taxonomic groups. This is where things get more complicated as we are reaching the boundaries of DNA technology and data analysis. Evaluation of microbial association networks is a topic still under development and no validated experiment for assessing microbial ecological network exists.
Because we will be able to know which taxonomic groups are present or absent in all our samples, one approach we intend to use is the analysis of co-occurrence, which is a long-term method to infer different types of interactions in ecological systems. Even though this method may not detect real biotic associations, it has proven to be suitable for getting an idea of microbiome complexity, which is a good start. We are still brainstorming other suitable approaches which may involve more intricated statistical methods of inference. More to come!
Photo credits: Oscar Aguinaga Vargas, Emily Waddell, Ross Barnett, Sam Rogerson
July 2022: Multi-functionality in ecological restoration
By Dr Emily Waddell, Woodland postdoctoral researcher
The aim of RestREco is to consider complexity, multi-functionality, and resilience as fundamental aims for restoration projects, rather than attempting to re-create specific reference ecosystems. The rationale for this is two-fold: (1) “pristine” reference ecosystems are hard to define, and (2) climate change is leading to a shifting baseline, and there is a need to restore ecosystems that are resilient to future pressures (i.e. environmental change). There is a large theoretical and conceptual literature that links ecosystem complexity to resilience and stability, to the supply of multiple functions and services, and to the conservation of biodiversity. But what a “complex ecosystem” looks like, and how complexity influences functions and resilience, remains largely unstudied in real-world landscapes.
Last year, we collected a whole suite of ecological data within each of our 132 grassland and woodland sites which will allow us to calculate different measures of ecological complexity (e.g. species richness, structural complexity, acoustic complexity, food web complexity). Ultimately, determining how ‘complex’ each individual grassland or woodland is. Now, in a subset of sites, we are collecting data on the next aim, ‘multi-functionality’, which requires more detailed work on a range of ecosystem functions (e.g. litter decomposition rates, pollination services, and herbivory and predation rates). These sites were selected to represent a gradient in complexity, to quantify if and how complexity supports net gains in the emergent functionality of ecosystems.
We are expecting complexity to enhance multiple ecosystem functions, such as decomposition, carbon capture and pollination, through more efficient resource capture and transfer, greater standing stocks and richness of producers and prey, and a diversity of interactions and niches. For example, the more species there are in an ecosystem, the higher the likelihood that multiple species perform similar functions, and so essential functions are maintained even if one species is lost from the ecosystem. This is known as functional redundancy and is a key mechanism linking complexity and resilience.
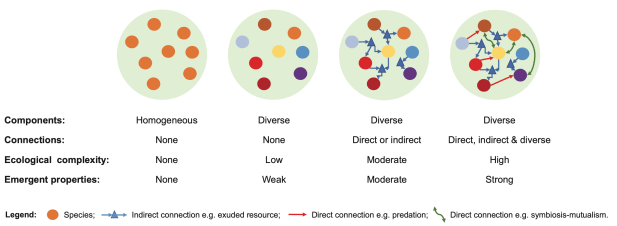
A conceptual diagram depicting increasing degrees of ecological complexity within a system (Figure 1 in Bullock et al. (2021) Ecography)
For more information on this topic, please see our recent peer-reviewed publication in Ecography, entitled “Future restoration should enhance ecological complexity and emergent properties at multiple scales”, and the references within.
From the field:
Starting ecosystem function measurements in Scottish Woodlands
By Ross Barnett, Woodland research technician
Our fieldwork this month has focused on quantifying four key ecosystem functions in our 18 woodlands: pollination, predation, herbivory and parasitism. Each has been investigated using a different set of observations and field experiments.
First up, is our pollination experiment. We chose the widespread creeping buttercup (Ranunculus repens) as the model species for this. The experiment simply involves finding a buttercup plant with two unopened flower buds. We cover one bud with a small organza bag, to stop pollinating insects accessing it, and the other bud is marked with red string, serving as a control. Over the following weeks, we returned to harvest the seed heads from all bagged and unbagged flowers, which will be dried in the lab before counting the number of seeds produced by each flower.
Next is our predation experiment using artificial caterpillars. Our field team of undergraduate students painstakingly crafted 1440 caterpillars using non-toxic green plasticine (yes, really!), which have been deployed in our woodlands, on tree branches and on the ground. Plasticine is an ideal material for this experiment because it is strong enough to hold the caterpillar shape, but soft enough that any bite marks from foraging insects, birds and rodents are easily identifiable. This is a fairly commonly used method to quantify relative levels of predation within habitats. After several days in the field, the caterpillars are collected, and the bite marks counted and identified to predator.






Clockwise from top left: Pollination exclusion experiment on creeping buttercup; pollinator of buttercup, Oedemera nobilis; predation of artificial caterpillar on branch by birds; predation of artificial caterpillar by slugs or snails; predation on ground by birds; predation by rodents.
To quantify insect herbivory on trees, we focused on our two most common groups of trees: oak and birch. At each site, we gathered branches from each species using telescopic pruning shears to make sure the leaves were out of reach from grazing deer or livestock. On each branch, the first ten fully developed leaves were chosen, and the percentage leaf area grazed was estimated by eye into categories of herbivory. The same observer estimated these measurements to keep it consistent across sites.
Finally, we have been gathering leaves with evidence of leaf-mining activity by insect larvae. The larvae of several beetles, flies, butterflies, and moths use leaf mines as a means of feeding and pupating with additional protection from predators. Many parasitic wasps, however, will lay an egg inside the leaf-mining larva, benefiting also from the protection of the mine, whilst the wasp larva feeds on the leaf-miner from the inside out (yikes!). Our collection of mined leaves is being kept in an insectary whilst we wait for either the emergence of an adult leaf miner or a parasitic wasp!







Clockwise from top left: Birch branch; Two leaf miners on a hawthorn leaf; Leaf miner on an oak leaf; Insect larvae eating an oak leaf; Two tiny larvae eating a birch leaf; Emily looking for leaf miners in a nettle leaf; Ross using telescopic pruning shears to cut down branches.
Ultimately, we will use these data to compare pollination rates, predation, herbivory, and parasitism within each woodland along our complexity gradient.
Doing your undergraduate project with RestREco!
By Will Crook, final year Ecology student at University of Stirling
I’m currently working on my dissertation investigating how biodiversity influences ecosystem function in Scottish woodlands. I was drawn to this topic as I feel there is a dire need to help mitigate the global biodiversity crisis and joining the RestREco project seemed a way I could be well applied, restoring ecosystems close to home.
When I started summer fieldwork in June, I was surprised as to how much there was in the woods that I hadn’t noticed before! Once time is spent looking for leaf miners or buttercups, everything else is revealed including cyan mushrooms, iridescent beetles, napping bumblebees, and cute sawfly caterpillars. Collecting data as part of a team is a feeling that you’d struggle to beat; working hard and getting things done, while still finding time to crack jokes and have a laugh. The jokes become necessary once it starts to rain, although oftentimes those are the most fun days. I’m excited to continue researching over the summer, my knowledge of the natural world is expanding, my efficiency with fieldwork is improving, and I am gaining an immunity to nettle stings.








Top to bottom and left to right: Ross looking at sawfly larvae on birch; Will, Emily, Ross and Dominic in a sycamore tree; Georgia with Teddy (the fieldwork dog); Forget-me-not flowers; Leaf beetle on willow; Two weevils on birch; Moth on a nettle; Emily, Georgia and Will ‘enjoying’ the Scottish summer weather.
All photos taken within our 18 subset woodland sites.
Photo credits: Emily Waddell, Ross Barnett, Will Crook
June 2022: National Trust and the RestREco project
By Ben McCarthy, Head of Nature Conservation & Restoration Ecology at National Trust
With the UN’s Decade of Ecosystem Restoration well underway and increasing recognition that Nature Based Solutions are crucial in meeting the twin climate and biodiversity crises just how should we achieve our international targets and start bending the curve to achieve environmental net gain?
The UK’s recent political path has thrown wide open how best to reconfigure a national approach to meeting obligations emerging from the post-2020 UN Biodiversity Framework such as 30×30. The UK’s departure from the EU and it’s powerful nature directives mean that how the UK and devolved nations deliver against international commitments is up for debate. Alongside this, emerging plans to deliver the UK Government’s ambitious 25 Year Plan for the Environment means this is an important moment in time to ensure a Green Brexit and, as the current incumbent at No 10 might say, ‘build back better’.
The Westminster Government’s response was set out in their Nature Green Paper and recent consultation on associated targets. Whilst in many ways these adopt a traditional approach to meeting our international biodiversity commitments with various targets for species, habitat and nature recovery networks it also throws wide open how best to recover nature and measure this success.
Whilst many commentators may assess the government’s plans as ‘work in progress’ there remains a more fundamental challenge for researchers, policy makers and practitioners of how to rebuild nature for a changing future. A challenge that should not be shied away from. This issue comes into sharp focus for the National Trust as we fully recognise, as a major landowner, the critical role land use and management will play in achieving our strategic priorities for Net Zero and Nature’s Recovery.

Ensuring our delivery realises our ambition and secures more resilient ecosystems will clearly be key to delivering our twin priorities. Yet blindly chasing indigenous reference states or pristine plant communities may not be the most appropriate approach to tackling the challenges of the Anthropocene. And so our collaboration with the RestREco team to investigate how to create the architecture and functionality from our degraded land. The real-life significance of such endeavours should not be underestimated – and not only to justify the £300M/yr agri-environment budget being asked of HM Treasury. Nature’s recovery is fundamental to society’s ability to meet the climate challenge and core to our charitable purposes.
So I remain open and excited about shining light on a new paradigm for target setting for nature’s recovery that is based on a greater understanding of characteristics of functionally intact systems for all our benefits.
Visit the National Trust’s website to find out more about their many restoration projects http://www.nationaltrust.org.uk
From the field:
Measuring complexity at National Trust’s Slindon Estate
By Ross Barnett, Woodland research technician
We recently visited National Trust’s Slindon Estate, in West Sussex, to conduct surveys of flora and fauna that will provide us with data to characterise the ecological complexity of the newly wooded areas within the estate. Slindon is one of two ‘rewilding wildcard’ sites for the RestREco project that have taken unique approaches towards woodland regeneration – in this case, excluding herbivores with a deer fence, and allowing seed dispersal and natural colonisation from the surrounding woodland. Our complexity measures will combine data from tree surveys, understorey plant communities and insect abundances, along with soundscape recordings from earlier in the spring, to see how this ‘wildcard’ compares to our more traditional woodland creation sites. Whilst sampling the lower branches of trees at Slindon, we noticed high numbers of the metallic green Phyllobius weevils (species TBC!), which often feed on the buds and leaves of willow trees (Salix spp.), which dominate the tree community at this site.




Clockwise from top: Young trees within deer fenced ‘wildcard’ woodland at Slindon Estate; farmland matrix surrounding woodland within Slindon Estate; Phyllobius weevil; Dr Matt Guy and Prof. Kevin Watts identifying ground flora in the field. Photo credits: Ross Barnett and Sam Rogerson (weevil).
April 2022: Exploring the complexity of grasslands
By Maico Geert Weites, Grassland research technician
Grasslands come in many shapes and sizes and the Southern English grasslands we study overwhelmingly comprise chalk grassland. Chalk grassland is a man-made habitat that was created by the logging of woodland by neolithic farmers in the chalk districts of England. This created an open steppe-like landscape. The shallow soils were often poorly suited for crop cultivation and were used as grazing grounds for cattle and sheep.
One may be under the impression that such infertile man-made landscapes are perhaps not that species-rich, however they are amongst the most biodiverse habitats in the UK and are essential for the survival of everything from Chalkhill Blue and Sainfoin Blunthorn Bee to Burnt-tip Orchid and Great Bustard.
The open plains gradually came under pressure from enclosure and agricultural intensification throughout the 19th and 20th centuries and many were either converted into arable land or were otherwise agriculturally improved to be more productive. Species-rich chalk grasslands made way for wheat and grazing grounds dominated by Perennial Ryegrass. The period between 1940 and 1984 saw a reduction of the British chalk grassland area by 80%. Looking at unimproved grasslands more broadly a decrease of 93% was observed between 1932 and 1984 in England and Wales. Many of the larger remaining areas of downland are now nature reserves or military training grounds.
These grasslands are of big cultural and conservation importance and provide a range of ecosystem services such as carbon storage. Conserving and restoring these grasslands demands knowledge in how to create resilient grasslands in the age of climate change and the biodiversity crisis.
Our 66 grassland study sites vary in age, from centuries-old old-growth grassland to grassland that has only been reverted from arable land within the last two years. This variation gives us a good insight on how species composition and overall complexity of sites vary with age.
Last year we conducted a series of surveys looking at a wide range of taxonomic groups to look at diversity and complexity by conducting pollinator surveys, invertebrate suction sampling, botany surveys, and taking soil cores to look at the soil microbiology. To give you an indication of the species richness of these fields, the number of vascular plant species recorded on the transects ranged from 11 to 53 species. Currently we are still processing many of last year’s invertebrate samples and we found lots of interesting stuff! Some highlights discovered during the microscope peeking include the larvae of the rare Rugged Oil Beetle stuck to the legs of solitary bees in whose nests they develop, to samples from a single site with 12 species of weevil.

Clockwise from top left: flower-rich chalk grassland on a Wiltshire study site; Chiltern Gentian (Gentianella germanica); a study site dominated by Rough Hawkbit (Leontodon hispidus); Dark Green Fritilary (Speyeria aglaja) on Dwarf Thistle (Cirsium acaule); a field site in Hampshire with lots of Pyramidal Orchid (Anacamptis pyramidalis); Burnt-tip Orchid (Neotinea ustulata); English Longhorn cow and calf and Stonehenge as seen from one of our field sites placing this man-made habitat in a historic context.
This year we will be conducting more in-depth surveys over several of our study sites, looking at the soundscape and the diets of the predatory invertebrates amongst others. In March have set up several experimental plots in which we sowed additional plant species and added additional carbon and we will be monitoring this over the coming years to see if and how this affects diversity and complexity. The coming field season is promising to be an intense but exciting one. Hopefully we will be able to collect some good quality data and enjoy the lovely landscapes and species that cross our path.

Clockwise from top left: Adonis Blue (Polyommatus bellargus) on its larval food plant Horseshoe Vetch (Hippocrepis comosa); Vestal Cuckoo Bee (Bombus vestalis) on Wild Thyme (Thymus drucei); Cistus Forester (Adscita geryon); a larva of the Black Oil Beetle (Meloe proscarabaeus); a larva of the Rugged Oil Beetle (Meloe rugosus) hitching a ride on the leg of a Common Furrow Bee (Lasioglossum calceatum); Heath Snail (Helicella itala), and Glutinous Earthtongue (Glutinoglossum glutinosum).
All photos taken within our 60 grassland sites.
Photo credits: Maico Geert Weites
March 2022: How to create complex woodlands
By Prof Kirsty Park, Principal Investigator
As outlined in February’s blog, the aim of RestREco is to identify factors influencing the trajectory of ecosystem complexity during restoration – in this blog we’ll take a look at one of the two habitats we are focussing on: woodland.
Woodland is one of the most biologically diverse systems on Earth, but forest systems have been severely affected by habitat loss and their cover has been reduced by ca. 50% worldwide in the last three centuries. Remaining woodlands are often highly fragmented and degraded, consisting of many relatively small and isolated patches immersed in a sea of agriculture or urban conurbation.









Whilst we know quite a bit about the effects of woodland loss and fragmentation on wildlife, we know much less about the effects of “putting it back” i.e. woodland creation. In part, this is because of the very long-time scales over which woodlands grow and develop. In addition, it takes time for species to arrive and establish in new habitats (sometimes referred to as colonisation credits). All this makes it very hard to conduct experiments on appropriate spatial and temporal scales. However, we can use a ‘natural experiment’ approach, which has the potential to overcome some of the challenges of landscape-scale studies. Natural experiments overlay an experimental design on an ecosystem where change or active manipulation has occurred or is planned, beyond the control of the researcher. In RestREco, we have selected secondary woodlands established on land formerly used for industry (‘brown field’ sites) or agriculture. We then selected sites from a gradient of surrounding woodland since this is likely to influence the speed at which species can colonise new woodlands. Because these are all sites of known planting age (10-60 years old), we can look at how these factors interact over time to influence the structure and function of the species assemblages at these sites, and ultimately ecological complexity.
At a subset of these sites we will also be testing the effects of thinning and stacking brash around young trees, to examine how management interventions influence ecological function and complexity. Thinning (selective removal of trees) is a commonly used forestry technique to improve the quality and growth of the remaining trees. We also know that many woodlands have high herbivory pressure due to deer grazing on tree seedlings and saplings, which reduces regeneration, a crucial component of forest ecosystems. We hope to deter deer browsing by stacking brash around young trees, as a protection from this disturbance. We will be examining the effects of these interventions on measures of complexity and ecological function over the next two years.
Braving the Scottish summer weather and waist high stinging nettles, the woodland team have completed surveys on ecosystem complexity in 30 woodlands across Central Scotland. Surveys of trees, flowering plants and invertebrates, along with audio recordings of birds and bats will provide data on structural complexity and biodiversity. By characterising each of these components of woodland complexity, we can determine how age, former land-use and woodland in the landscape influences complexity. This summer we will continue these surveys in 30 more woodlands in the midlands of England, around the National Forest (project partners of RestREco), as well as carrying out more in-depth surveys into measures of ecosystem function in 18 of our Scottish woodlands.







Planting trees is just one way of creating woodland – recently, there has been considerable focus on natural colonisation, a process by which woodlands establish naturally from local seed dispersion. Both methods have their place depending on the local circumstances, but what is clear is that whichever method (or mix of methods) is used, it is more important than ever that the right mix of trees is planted (or natural colonisation facilitated) in the right places to ensure the multiple benefits we need from our landscapes in the future.
Other information and related projects:
Watts K, Whytock RC, Park KJ, Fuentes-Montemayor E, Macgregor NA, Duffield S & McGowan PJK (2020) Ecological time lags and the journey towards conservation success. Nature Ecology & Evolution
Woodland Creation and Ecological Networks, the WrEN project: https://www.wren-project.com/
All photos taken within our 30 woodland sites across Central Scotland
Photo credits: Dr Emily Waddell, Sam Rogerson and Ross Barnett
February 2022: Restoring Resilient Ecosystems
By Prof Jim Harris, Consortium Lead Principal Investigator
We are facing the Sixth Mass Extinction, coupled with rapid Climate Change and large-scale transformation of natural ecosystems for agriculture, mineral extraction and urbanisation. Although it comes as no surprise to many that we are utterly dependant on the biosphere for our continued existence, only in recent years have world governments become focussed on these challenges, and the need for urgent action.
Part of the solution is the need to ensure our ecosystems are “resilient”, so that they can continue to function in the face of the acute upswing in extreme events, such as droughts, flooding and wildfires. Such ‘Nature based solutions’ are beginning to gain widespread traction.

The United Nation has this year launched “The Decade on Ecosystem Restoration”, as a way of galvanising action to combat biodiversity loss, but also as a means of sequestering carbon. But what do we mean by “ecosystem restoration” and how can we assess if the restoration has been successful? – and why do we need this research programmes such as ours? We intend to measure biodiversity, architecture and multifunctionality in ecosystems in different stages of transition from a degraded state, identify determinants and measures of complexity, and seek signals of emergent properties – especially resilience to perturbation. We have chosen grasslands and woodlands, being two major habitat types targeted for restoration programmes. Further to this we shall explore how approaches to accelerating re-integration of systems may affect emergent properties. In summary, we propose to move restoration science forward, but considering complexity and resilience as fundamental aims for restoration projects, rather than attempting to re-create specific target ecosystems.
But surely restoration involves just “putting things back to how they were before”? If only it was that easy! Climate change has changed the “biophysical envelope” in many regions of the world – with variations in temperature and precipitation not seen for millennia, if not longer, and at a pace that is stretching, if not beyond the normal “plasticity” of ecosystems’ adaptive or evolutionary responses. Couple this with other anthropogenic pressures to systems, including intensifying species extinction, “going back” is no longer an option in many circumstances, and certainly doesn’t guarantee that it will be resilient in the future.
So, if we can’t simply aim to make systems the way they used to be, and recognising our current systems are themselves under pressure, how are we going to make decisions as to what kind of targets we should aim for? That is where our project is trying to find answers.
For a complex structure, such as the many habitats we benefit from, to be considered as a system, it needs to have a number of characteristics – diversity, complexity, function, and dynamic emergent properties – particularly, resilience. We aim to uncover the relationship between these characteristics on a range of sites under restoration management programmes. In this way the targets that we aim for may not need to be precisely those that existed in the past in terms of their species components, but rather have similar complexity, function and emergence – sometimes formulated as “same play, different actors”.

We shall be looking at everything from the soil microbial community, vegetation, invertebrates, birds and bats, trying to detect signals of complexity in structure and function, and what factors influence the trajectory of complexity during restoration for management. What is more, we will tie those signals to emergent properties of those systems.
We hope that this will give us another management tool alongside the tried and trusted methods based on ecosystems that we already recognise, as this approach applies as much to them as to any newly emergent assemblages of plants and animals which are beginning to appear, to give us confidence that we are making progress in securing an enduring future for our biodiversity and the ecosystems upon which we depend.
